作者简介:丁峰(1982-),男,高级工程师,从事含能功能材料合成及性能研究。E-mail: m-top@163.com 通信作者:陆婷婷(1986-),女,博士,副研究员,从事含能功能材料合成及性能研究。E-mail: 252525tt@163.com
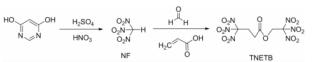
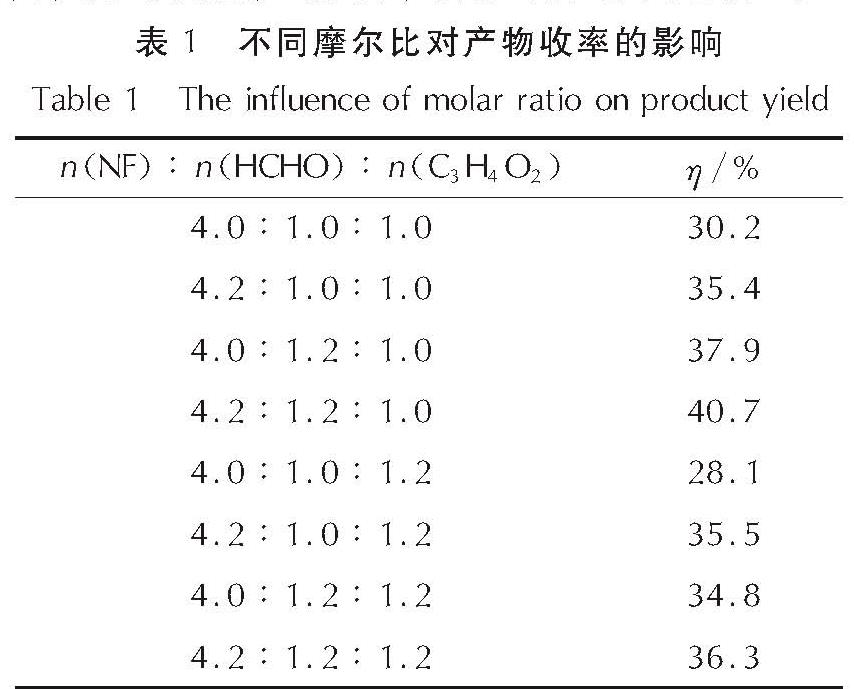
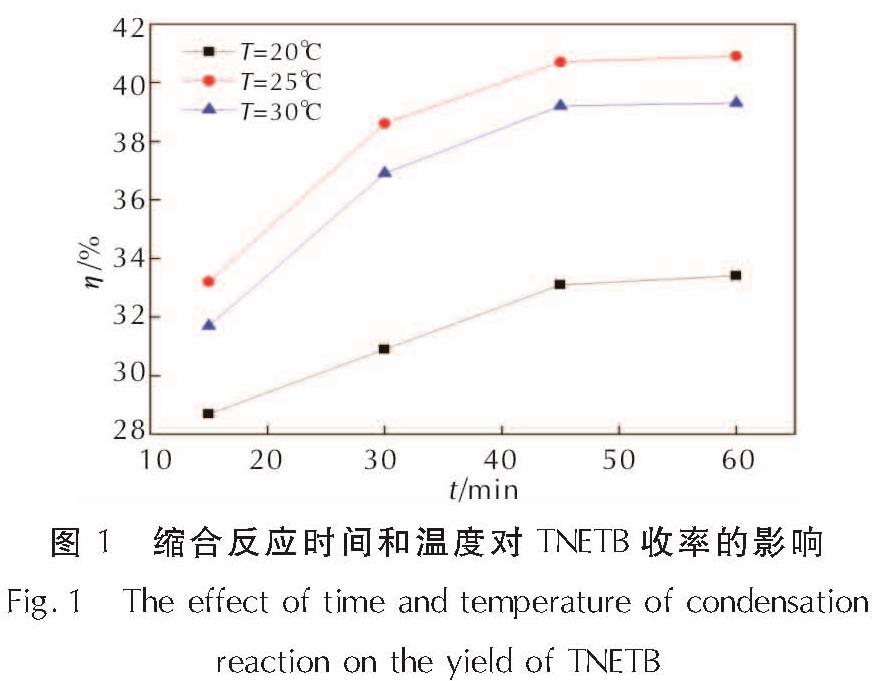
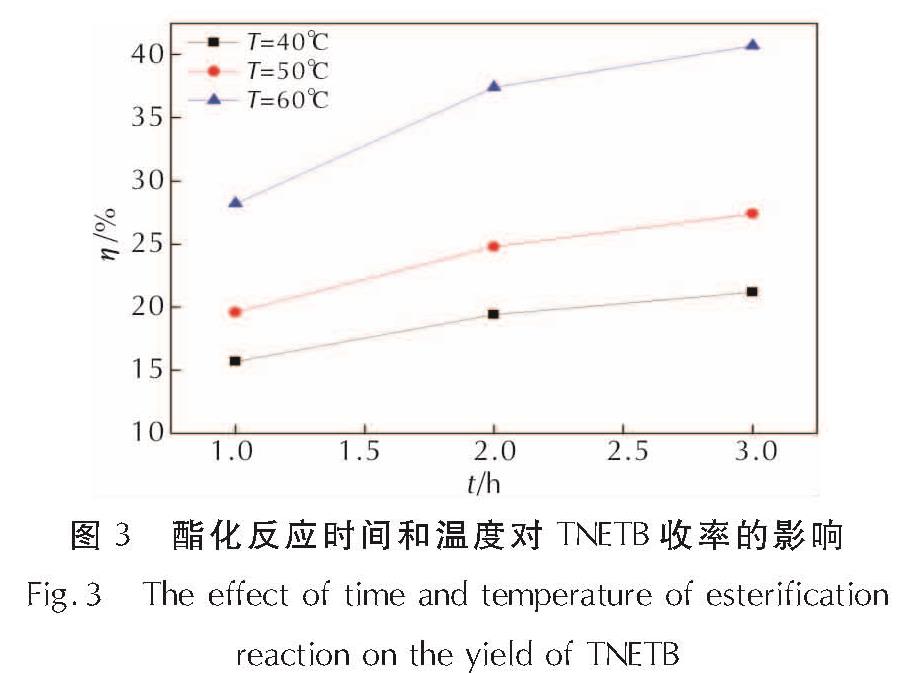
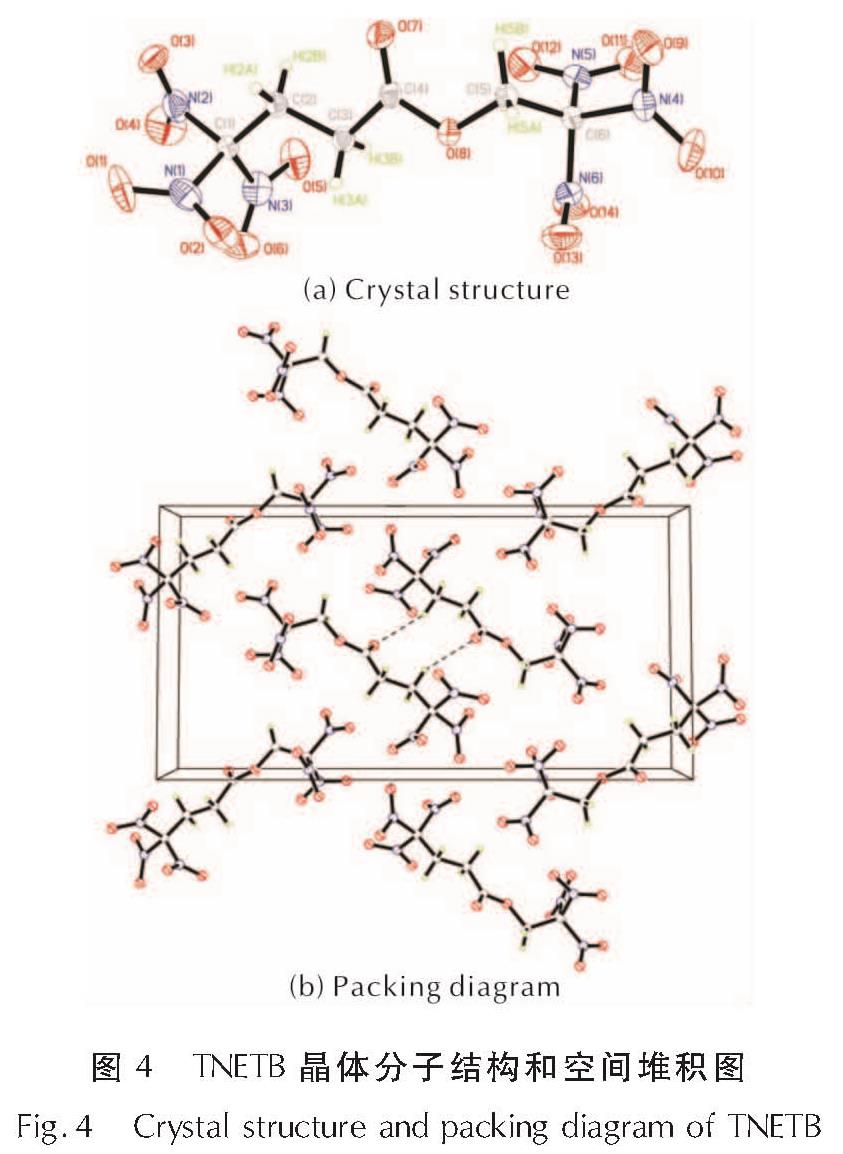
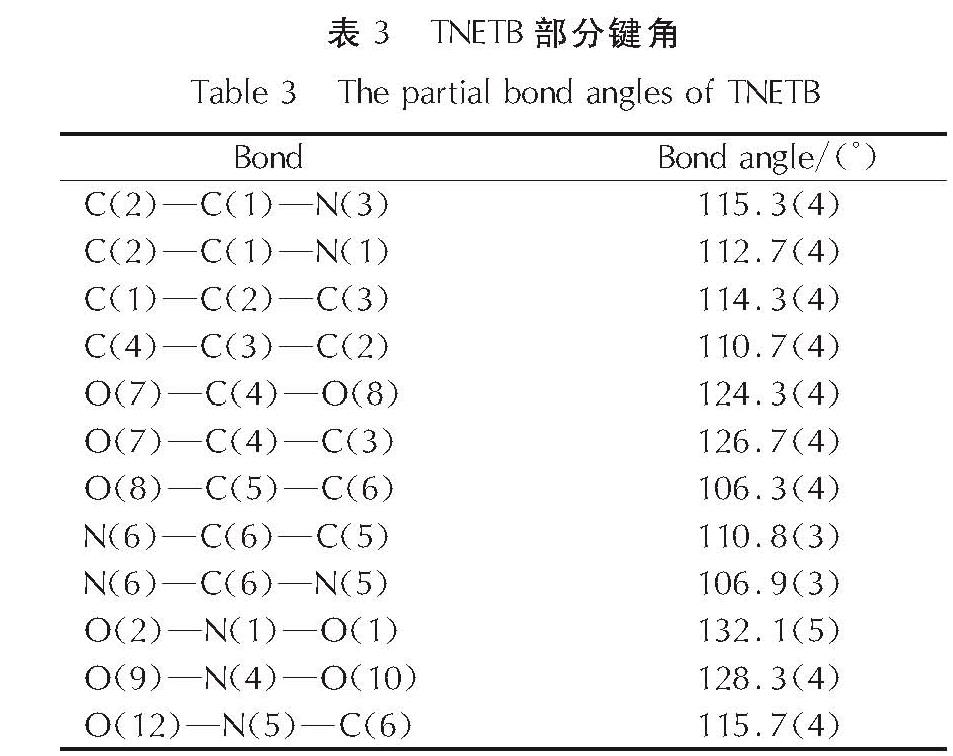
为了研究硝仿系炸药的合成,以4,6-二羟基嘧啶为硝化原料制备硝仿,然后合成4,4,4-三硝基丁酸-2,2,2-三硝基乙酯(TNETB); 采用红外光谱、核磁共振波谱、质谱以及元素分析等对TNETB进行了结构表征; 考察了物料比、反应温度和反应时间对产物收率的影响,以甲醇为溶剂培养了TNETB单晶,并采用X-射线单晶衍射进行晶体结构测定。结果 表明,制备TNETB的最佳反应条件为:硝仿、甲醛水溶液和丙烯酸的摩尔比为4.2:1.2:1.0,无需溶剂,缩合反应时间45min,反应温度为25℃; 加成反应时间为60min,反应温度为50℃; 酯化反应时间为60min,反应温度为50℃; TNETB的收率为40.7%,纯度为98.7%; TNETB晶体属于单斜晶系,P2(1)/n空间群,晶体学参数为:a=5.8799(13)Å,b=21.801(5)Å,c=11.220(2)Å,V=1434.1(5)Å3,Z=4,DC=1.789g/cm3,μ=0.180mm-1,F(0 0 0)=784,R1=0.0960,ω R2=0.2857; TNETB分子间的氢键和范德华力降低了硝仿基团的引入对分子稳定性和安全性的影响,表明TNETB具有较高的安全性。
In order to develop nitroform explosive, nitroform was synthesized from 4,6-dihydropyrimidine and continued to prepare 2',2',2'-trinitroethyl-4,4,4-trinitrobutyrate(TNETB). The structure of TNETB was characterized by 1H NMR, 13C NMR, IR, MS and elemental analysis. The effects of substrate ratio, reaction temperature and reaction time on product yield were investigated. The single crystal of TNETB was cultivated from methanol and was characterized by single crystal X-ray diffraction. The results show that when n(NF): n(HCHO): n(C3H4O2)is 4.2:1.2:1.0, the reaction temperature is 50℃,the condensation, the addition and the esterification time are 45 min, 60 min and 60 min, respectively, the yield of TNETB was 40.7% with purity of 98.7%. The crystal belongs to monoclinic system, space group P2(1)/n, with crystal parameters of a=5.8799(13)Å,b=21.801(5)Å,c=11.220(2)Å,V=1434.1(5)Å3,Z=4,DC=1.789g/cm3,μ=0.180mm-1,F(0 0 0)=784,R1=0.0960,ω R2=0.2857. The hydrogen bond and Van der Waals force reduce the impact of molecular stability from the introduction of nitroform group, and increase the safety of TNETB.