作者简介:赵坤(1981-),女,博士,讲师,从事纳米催化剂、电解新材料等研究工作。E-mail:47909663@qq.com
(二硝基甲基)氧化呋咱铵盐的合成、晶体结构及性能] 赵 坤1,黄海丰2,3,石亚猛2,杨 军2,李红丽4 (1.湖北文理学院 食品科学技术学院,湖北 襄阳 441053; 2. 中国科学院上海有机化学研究所 中国科学院能量调控材料 重点实验室,上海 200032; 3. 中国科学院绿色过程制造创新研究院,北京 100190; 4. 重庆医药高等专科学校 医学技术学院,重庆 401331)
(1. School of Food Science and Technology, Hubei University of Arts and Science,Xiangyang Hubei 441053, China; 2. CAS Key Laboratory of Energy Regulation Materials, Shanghai Institute of Organic Chemistry,Chinese Academy of Sciences, Shanghai 200032, China; 3. Innovation Academy for Green Manufacture, Chinese Academy of Sciences,Beijing 100190, China; 4. College of Medical Technology, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China)
organic chemistry; energetic ionic salt; furoxan; heterocycle
DOI: 10.14077/j.issn.1007-7812.202008001
备注
作者简介:赵坤(1981-),女,博士,讲师,从事纳米催化剂、电解新材料等研究工作。E-mail:47909663@qq.com
为了获得具有更高能量的含能化合物,以3,4-双(氯肟)氧化呋咱为原料,经过N2O5/N2O4硝化和乙腈中酸化-酸碱中和两步反应制备得到了3,4-双(二硝基甲基)氧化呋咱铵盐; 采用核磁共振、元素分析、单晶X-射线衍射对3,4-双(二硝基甲基)氧化呋咱铵盐进行了结构表征; 并用TG-DSC测定了该化合物的热稳定性,采用理论计算的方法获得了其生成焓数据; 基于单晶密度和理论生成焓,采用Kamlet-Jacob方程计算了该化合物的爆速和爆压。结果 表明,3,4-双(二硝基甲基)氧化呋咱铵盐两步反应收率分别为73%和75%,晶体密度为1.783g/cm3,热分解温度为131℃,生成焓为-123.5kJ/mol,爆速为8802m/s,爆压为34.2GPa,表明它是一种高氧平衡、高能量的含能化合物。
To achieve energetic compounds with higher energy-density levels, the ammonium salt of 3,4-bis(gem dinitro)furoxan was synthesized from 3,4-bis(chloroxime)furoxan via N2O5/N2O4 nitration followed by a acidification-neutralization process in acetonitrile. The structure of diammonium 3,4-bis(dinitromethyl)furoxanate was confirmed by nuclear magnetic resonance, elemental analysis and single crystal X-ray diffraction. Its thermal stability was measured by TG-DSC. Its enthalpy of formation was theoretically calculated. Based on the crystal density and enthalpy of formation, the detonation velocity and detonation pressure of the title compound were calculated. The results show that the yields of two steps are 73% and 75%, respectively. Its crystal density, thermal decomposition temperature, enthalpy of formation, detonation velocity and detonation pressure are 1.783g/cm3, 131℃, -123.5kJ/mol, 8802m/s and 34.2GPa, respectively, showing that diammonium 3,4-bis(dinitromethyl)furoxanate is a high energetic compound with high oxygen balance.
引言
发展新型含能化合物始终是含能材料领域的研究热点之一,其为武器弹药实现“高效毁伤”和“远程打击”的重要能量来源[1-2]。因此,含能化合物的发展方向之一是设计并合成具有更高能量的含能化合物,同时也希望其具备高密度、良好的热稳定性、低感度等特点[3-4]。
含能离子盐是由含能阴离子和含能阳离子组成的一类离子型含能化合物。设计并合成新型含能离子盐是当前发展新型含能化合物的重要技术途径,这是因为含能离子盐通常热稳定好和感度低,且易于通过阴阳离子的单独修饰来调控其性能。富氮杂环结构如呋咱、氧化呋咱、三唑、四唑等被广泛用作含能离子盐阴离子的骨架结构,其中氧化呋咱环具有最高的氧平衡,非常适合用于设计高能化合物 [5-6]。
二硝基甲基氧平衡高且含有强酸性位点,3,4-双(二硝基甲基)氧化呋咱可以与富氮有机碱反应制备相应的富氮含能离子盐。2016年,He等[7]报道了3,4-双(二硝基甲基)氧化呋咱钾盐。同年,Zhai等[8]以3,4-双(二硝基甲基)氧化呋咱钾盐为原料,水相中经过酸化-乙醚萃取-酸碱中和制备了3,4-双(二硝基甲基)氧化呋咱肼盐。但是,乙醚对3,4-双(二硝基甲基)氧化呋咱的萃取效率非常低。本研究对3,4-双(二硝基甲基)氧化呋咱含能离子盐的制备方法进行了改进,发展了有机溶剂中酸化-酸碱中和制备3,4-双(二硝基甲基)氧化呋咱含能离子盐的方法,成功制备了3,4-双(二硝基甲基)氧化呋咱铵盐(化合物6),并对其进行了结构表征和性能研究。
1 实 验
2 结果与讨论
3 结 论
(1)以3,4-双(氯肟)氧化呋咱为原料,经过N2O5/N2O4硝化和KI脱氯制备得到了3,4-双(二硝基甲基)氧化呋咱钾盐,收率73%。并通过缩短硝化反应时间,首次分离并鉴定了氯硝基甲基中间体的单晶结构。
(2)采用乙腈中酸化-酸碱中和制备含能离子盐的新方法,并成功制备了(二硝基甲基)氧化呋咱铵盐,收率75%,其晶体密度为1.783g/cm3。
(3)3,4-双(二硝基甲基)氧化呋咱铵盐的热稳定性(Td)为131℃,其理论生成焓为-123.5kJ/mol。采用Kamlet-Jacob方程计算得到其爆速为8802m/s,爆压为34.2GPa。表明该化合物是一种高氧平衡、高能量的含能化合物。
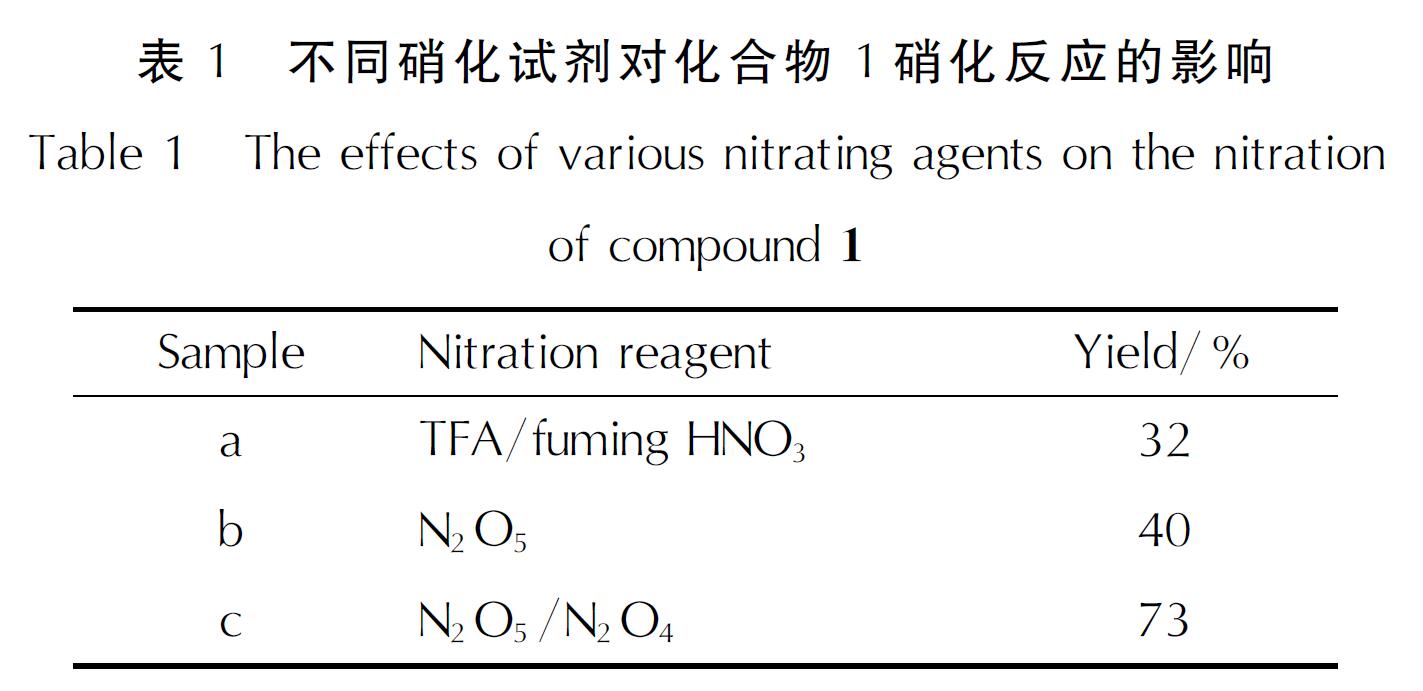
2.1 3,4-双(二硝基甲基)氧化呋咱铵盐(化合物6)的合成机理2.1.1 化合物4的合成条件优化文献中常用的氯肟底物硝化试剂为三氟乙酸酐(TFA)/HNO3[9-10]、N2O5[11-12]。本研究首先使用三氟乙酸酐/HNO3、N2O5对化合物1进行了硝化,结果列于表1中。表1中样品a为0.8g化合物1,5mL三氟乙酸酐,2.7mL 95%的发烟硝酸; 样品b为1.5g化合物1,5.0g N2O5; 样品c为6g化合物1,20g N2O5, 4g N2O4。
从表1可以看出,使用三氟乙酸酐/发烟HNO3为硝化试剂时收率为32%,而使用N2O5为硝化试剂时收率提高至40%。此前的研究中,采用N2O5/N2O4组合硝化剂有利于提高氯肟硝化的收率[13]。为了提高化合物1的硝化收率,使用N2O5/N2O4组合硝化剂对化合物1进行了硝化,收率提高至73%。
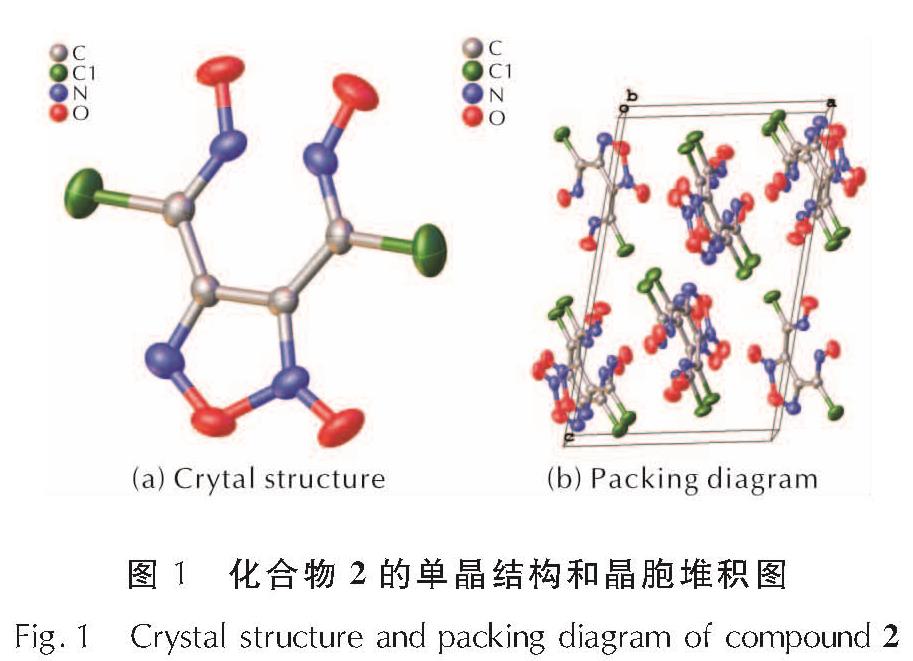
硝化3,4-双(氯肟)氧化呋咱(化合物1)的反应中间体是化合物2,通过缩短硝化反应时间分离出了该中间体,并采用单晶X-射线对其结构进行了确证,单晶结构如图1所示。硝化反应在0℃进行10min后用冰水淬灭反应,化合物2以沉淀的形式从水相中析出。
使用N2O5/N2O4组合硝化剂时,3,4-双(氯肟)氧化呋咱(化合物1)硝化反应收率高的可能原因为:N2O4的氧化性较强,会加速肟被氧化成亚硝基的速率。而N2O5则更容易对氯硝基甲基进行硝化,从而使N2O5/N2O4组合硝化剂实现了收率的提升。
2.1.2 由化合物4制备化合物6的合成机理及条件优化由钾盐制备相应含能离子盐的常规制备方法是将钾盐分散于水中后进行酸化,经过萃取后再与有机碱进行酸碱中和反应制备含能离子盐。本研究首先尝试了上述方法,将化合物4分散于水中后用盐酸进行酸化,然后分别尝试了乙酸乙酯、乙醚、二氯甲烷等常用萃取溶剂,其中仅乙酸乙酯可较为有效地将化合物5萃取至有机相中,而其他溶剂则均萃取效率极低。乙酸乙酯相经饱和食盐水洗涤、干燥、浓缩得液体,对其进行核磁分析发现其含大量乙酸。由于乙酸沸点高,而中间体5稳定性较差,因此,无法通过升温浓缩来完全除去乙酸。乙酸乙酯相经干燥后,通入干燥的氨气,浓缩后进行核磁表征,所得的氢谱和碳谱如图2所示。氢谱中δ为1.82和碳谱中δ为23.8、176.9的峰对应于乙酸铵的信号,这与文献中乙酸铵的化学位移相似[14]。
图2 水相中酸化萃取制备的化合物6的氢谱和碳谱
Fig.2 1H NMR and 13C NMR spectra of compound 6 prepared by acidification in water考虑到KCl在有机溶剂中的溶解度较小,25℃下KCl在乙腈中的溶解度为2.4×10-3g/100g。将钾盐4分散于乙腈中后,向其中通入过量干燥的氯化氢气体。由于HCl的酸性强于化合物5的酸性,依据“强酸制弱酸”原理,黄色钾盐4溶解并析出白色KCl沉淀,得到中间体5的乙腈溶液,该溶液经过无水MgSO4干燥后过滤,室温减压浓缩得中间体5。对乙腈相进行干燥可以降低溶液中的水分含量,达到减少中间体中HCl含量的目的,最终,降低目标产物中的盐酸盐即氯化铵含量。将中间体5分散于乙腈中,通入过量的干燥氨气,减压浓缩即得产物6粗品,其经乙醇/水重结晶得纯品,合成路线如下:
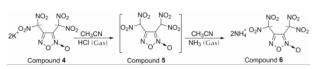 2.2 化合物6的晶体结构
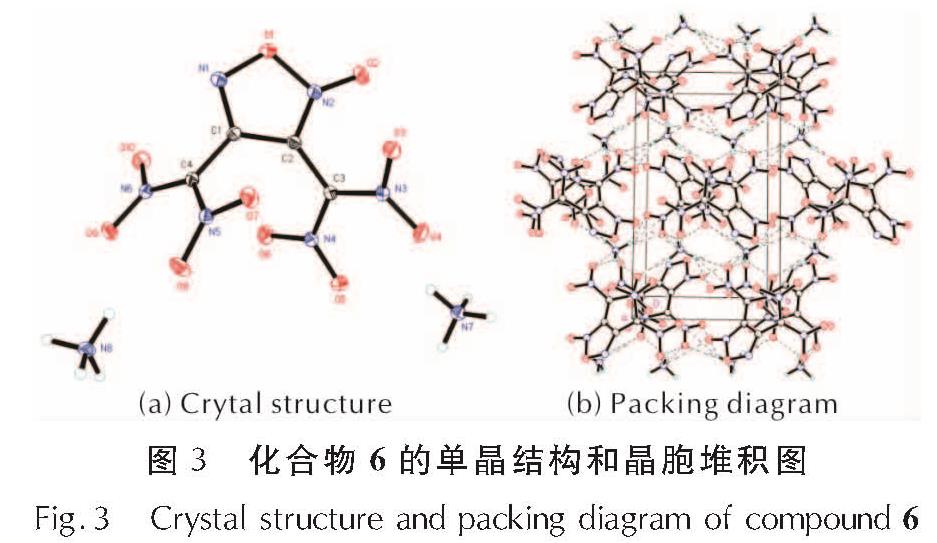
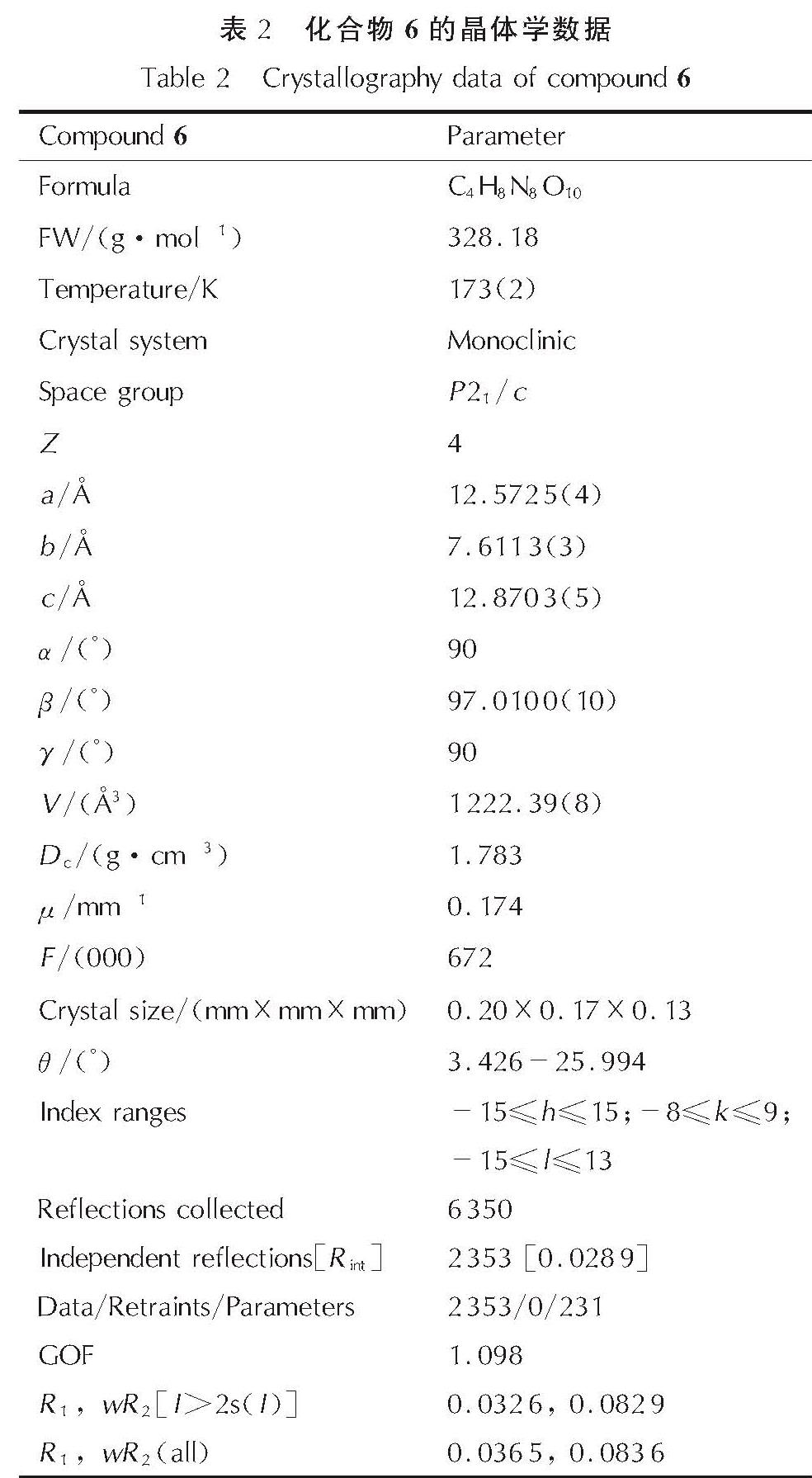
2.2 化合物6的晶体结构将适量化合物6溶解于甲醇和水的混合液中,室温下缓慢挥发溶剂得到适合于单晶X-射线衍射的晶体并进行晶体结构测定,其单晶结构如图3所示,其晶体学数据如表2所示。该化合物的CCDC号为1988948,晶体数据可以通过https://www.ccdc.cam.ac.uk获得。
化合物6属于单斜晶系P21/c空间群,每个晶胞中包含4个阴阳离子对,晶体密度为1.783g/cm3(173K)。氧化呋咱环上的氮氧键O1—N2的键长为1.443Å,O1—N1的键长为1.378Å,氧化呋咱环外的N2—O1的键长为1.226Å,氧化呋咱环外O2侧的氮氧键长于另外一侧的氮氧键。硝基上N—O键的平均键长为1.247Å。由于两个二硝基甲基间的距离较近,导致它们与氧化呋咱环间形成了较大的扭曲角(C1—C2—C3—N4=-51.25°; C2—C1—C4—N6=-119.93°)。
2.3 化合物6的热稳定性化合物6的TG-DSC曲线如图4所示。
从图4可以看出,该化合物加热过程中不经过熔化过程,而是直接分解,其分解分为两个阶段:第一阶段放热量较小,放热峰较宽,热分解峰温为131.0℃; 第二阶段放热峰尖锐,热分解峰温为163.2℃。
2.4 化合物6的理化和爆轰性能采用文献[15]的方法计算得到化合物的晶格能为1301.3kJ/mol,铵根离子生成焓为635.8kJ/mol,3,4-双(二硝基甲基)氧化呋咱双电荷阴离子的生成焓为-93.8kJ/mol[7],进一步根据该化合物的晶体密度(1.783g/cm3)和Born-Harber循环按公式(1)计算出化合物6的生成焓为-123.5kJ/mol。
ΔHf(ionic salt, 298K)=ΔHf(cation, 298K)+
ΔHf(anion, 298K)-ΔHL(1)
式中:ΔHf(ionic salt, 298K)为盐的生成焓,kJ/mol; ΔHf(cation, 298K)为阳离子的生成焓,kJ/mol; ΔHf(anion, 298K)为阴离子的生成焓,kJ/mol; ΔHL为盐的晶格能,kJ/mol。
基于晶体密度和计算生成焓,根据Kamlet-Jacob方程(2)和(3)[16]计算了化合物6的爆速和爆压,分别为8802m/s和34.2GPa,见表3,其爆轰性能与RDX(D=8841m/s; p=34.8GPa)相当。
D=1.01(NM^-1/2Q1/2)1/2(1 + 1.30ρ)(2)
p=1.558ρ2M^-1/2Q1/2(3)
式中:D为爆速,km/s; p为爆压,GPa; ρ为炸药的密度,g/cm3; Q为每克炸药爆炸化学能,即单位质量最大爆热,kJ/kg; N为每克炸药爆轰时生成气态产物的物质的量,mol/g; M^-为气体爆轰产物平均摩尔质量,g/mol。
化合物6的摩擦感度和撞击感度分别为72N和3J,其安全性显著低于RDX(FS=120N; IS=7.5J)。
- [1] 王恒生, 张国军, 程艳婷, 等. 固体推进剂中新型含能材料研究进展[J]. 化工科技, 2012, 20(1): 76-80.
- [2]王文俊. 新型含能材料及其推进剂的研究进展[J]. 推进技术, 2001, 22(4): 269-275.
- [3]田均均, 张庆华, 李金山. 含能分子合成最新进展[J]. 含能材料, 2016,24(1): 1-9.
- [4]TANG Y, DHARAVATH S, GREGORY H I, et al. Nitramino- and dinitromethyl-substituted 1,2,4-triazole derivatives as high-performance energetic materials[J]. Chemistry—An European Journal, 2017, 23: 9185-9191.
- [5]黄海丰, 孟子晖, 周智明, 等. 含能盐和含能离子液体[J]. 化学进展, 2009,21(1): 152-163.
- [6]YIN P, ZHANG J, MITCHELL L A, et al. 3,6-Dinitropyrazolo[4,3-c]pyrazole-based multipurpose energetic materials through versatile N-functionalization strategies[J]. Angewandte Chemie International Edition, 2016, 55: 12895 -12897.
- [7]HE C, SHREEVE J M. Potassium 4,5-bis(dinitromethyl)furoxanate: a green primary explosive oxygen balance[J]. Angewandte Chemie International Edition, 2016, 55: 772-775.
- [8]ZHAI L, QU X, WANG B, et al. High energy density materials incorporating 4,5-bis(dinitromethyl)-furoxanate and 4,5-bis(dinitromethyl)-3-oxy-furoxanate[J]. ChemPlusChem, 2016, 81(11): 1156-1159.
- [9]LEI C, YANG H, CHENG G. New pyrazole energetic materials and their energetic salts: combining the dinitromethyl group with nitropyrazole[J]. Dalton Transaction, 2020, 49: 1660-1667.
- [10]YU Q, CHINNAM A K, YIN P, et al. Finding furoxan rings[J]. Journal of Materials Chemistry A, 2020, 8: 5859-5864.
- [11]ZHAI L, FAN X, WANG B, et al. A green high-initiation-power primary explosive: synthesis, 3D structure and energetic properties of dipotassium 3,4-bis(3-dinitromethylfurazan-4-oxy)furazan[J]. RSC Advances, 2015, 5: 57833-57841.
- [12]YU Q, IMLER G H, PARRISH D A, et al. N,N[WTBZ]'[WTB5]-Methylenebis(N-(1,2,5-oxdiazol-3-yl)nitramide)derivatives as metal-free green primary explosives[J]. Dalton Transaction, 2018, 47: 12661-12666.
- [13]LI Y, HUANG H, SHI Y, et al. Potassium nitraminofurazan derivatives: potential green primary explosives with high energy and comparable low friction sensitivies[J]. Chemistry—An European Journal, 2017, 23: 7353-7360.
- [14]LOU C, QIN S, ZHANG S, et al. Non-redox metal ions promoted oxidative dydrogenation of saturated C—C bond by simple Pd(OAc)2 catalyst[J]. Catalysis Communication, 2017, 90: 5-9.
- [15]JENKINS H D B. Lattice potential energy estimation for complex ionic salts from density measurements[J]. Inorganic Chemistry, 2002, 41: 2364-2367.
- [16]KAMLET M J, JACOBS S J. Chemistry of detonations. I. A simple method for calculating detonation properties of C-H-N-O explosives[J]. Journal of Chemical Physics, 1968, 48: 23-35.
- [17]LIU Y, HE C, TANG Y, et al. Tetrazolyl and dinitromethyl groups with 1,2,3-triazole lead to polyazole energetic materials [J]. Dalton Transactions, 2019, 48: 3237-3242.
1.1 试剂与仪器3,4-双(氯肟)氧化呋咱(化合物1)根据文献[7]的方法制备。
Mercury VX 300核磁共振仪,美国瓦里安公司; Agilent 400 MHz核磁共振仪,美国安捷伦科技有限公司; Vario EL Ⅲ型有机微量元素分析仪,德国Elementar公司; STA449F3型热重-差示量热扫描仪,德国耐驰公司; BFH-10撞击感度仪和FSKM-10摩擦感度仪,美国爱迪赛恩公司; SMART APEX-II CCD单晶X-射线衍射仪,德国Bruker公司。
1.2 实验过程以3,4-双(氯肟)氧化呋咱(化合物1)为原料,经过硝化、有机溶剂中酸化、酸碱中和制备得到了3,4-双(二硝基甲基)氧化呋咱铵盐(化合物6),合成路线如下:
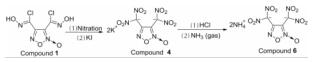 1.2.1 3,4-双(氯亚硝基甲基)氧化呋咱(化合物2)的合成
1.2.1 3,4-双(氯亚硝基甲基)氧化呋咱(化合物2)的合成冰盐浴冷却下,于25mL单口烧瓶中将三氟乙酸酐(5mL)和95%发烟硝酸(2.7mL)混合,待混酸的温度降至0℃后,向其中滴加3,4-双(氯肟)氧化呋咱(0.8g)的氯仿溶液(7mL),维持体系温度0~5℃反应10min。将反应液倒入70mL冰水中淬灭,析出黄色固体,过滤、水洗、自然干燥得产物336mg,收率42%。
1.2.2 3,4-双(二硝基甲基)氧化呋咱钾盐(化合物4)的合成化合物4的合成路线如下:
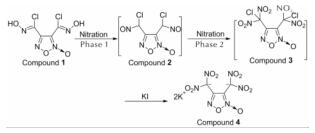
于单口烧瓶中加入200mL氯仿,冰盐浴冷却至0℃。向其中加入N2O5(20.0g)的氯仿溶液(100mL),再加入N2O4(4.0g)。然后,加入3,4-双(氯肟)氧化呋咱(化合物1, 6.0g),5min后撤冰盐浴,自然升温至室温下反应40min。冰水淬灭,分离氯仿相并用氯仿萃取2次,合并有机相,饱和NaCl洗涤3次,有机相经无水MgSO4干燥后过滤,滤液浓缩至约10mL溶剂。向其中加入90mL KI的甲醇溶液(12.0g KI/90mL甲醇),室温搅拌12h,过滤、甲醇洗涤、晾干得黄色固体6.8g,收率为73%。
13C NMR(100MHz),δ: 152.1, 123.3, 120.7, 112.1。元素分析(C4K2N6O10,%):理论值,C 12.98, H 0, N 20.70; 实测值,C 12.61, H<0.3, N 20.35。HPLC纯度为99.4%。
1.2.3 3,4-双(二硝基甲基)氧化呋咱铵盐(化合物6)的合成化合物6的合成路线如下:
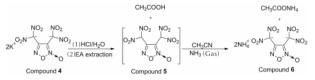
将0.5g 3,4-双(二硝基甲基)氧化呋咱钾(化合物4)盐悬浮于15mL乙腈中,搅拌下向其中通入过量干燥的氯化氢气体,立即产生白色沉淀,搅拌20min后使用无水MgSO4进行干燥,过滤、浓缩后向残留物分散于乙酸乙酯中,然后在搅拌下向其中通入过量的干燥氨气,析出黄色沉淀,过滤后用乙醇/水重结晶得产物332mg,收率为75%。
1H NMR(400MHz),δ: 7.23; 13C NMR(100MHz):152.3,123.7,120.0,112.4。元素分析(C4H8N8O10,%):理论值,C 14.64, H 2.46, N 34.15; 实测值,C 14.26, H 2.57, N 33.82。
1.3 性能测试采用热重-差示量热扫描仪(TG-DSC)在5K/min的升温速率下测试3,4-双(二硝基甲基)氧化呋咱铵盐的热稳定性,氮气氛围,常压,温度范围为40~400℃; 基于Born-Harber循环计算3,4-双(二硝基甲基)氧化呋咱铵盐的生成焓; 采用Kamlet-Jacob方程计算3,4-双(二硝基甲基)氧化呋咱铵盐的爆轰性能; 采用撞击感度仪(落锤质量2kg,药量200mg)和摩擦感度仪(药量20mg)测试3,4-双(二硝基甲基)氧化呋咱铵盐的撞击感度和摩擦感度。