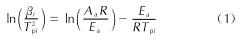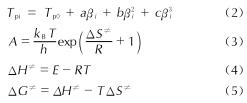作者简介:霍欢(1984-),女,副研究员,从事含能材料合成研究。E-mail: huohuan-234@163.com 通信作者:毕福强(1982-),男,副研究员,从事含能材料合成研究。E-mail: bifuqiang@gmail.com
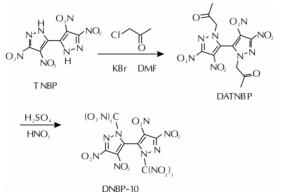
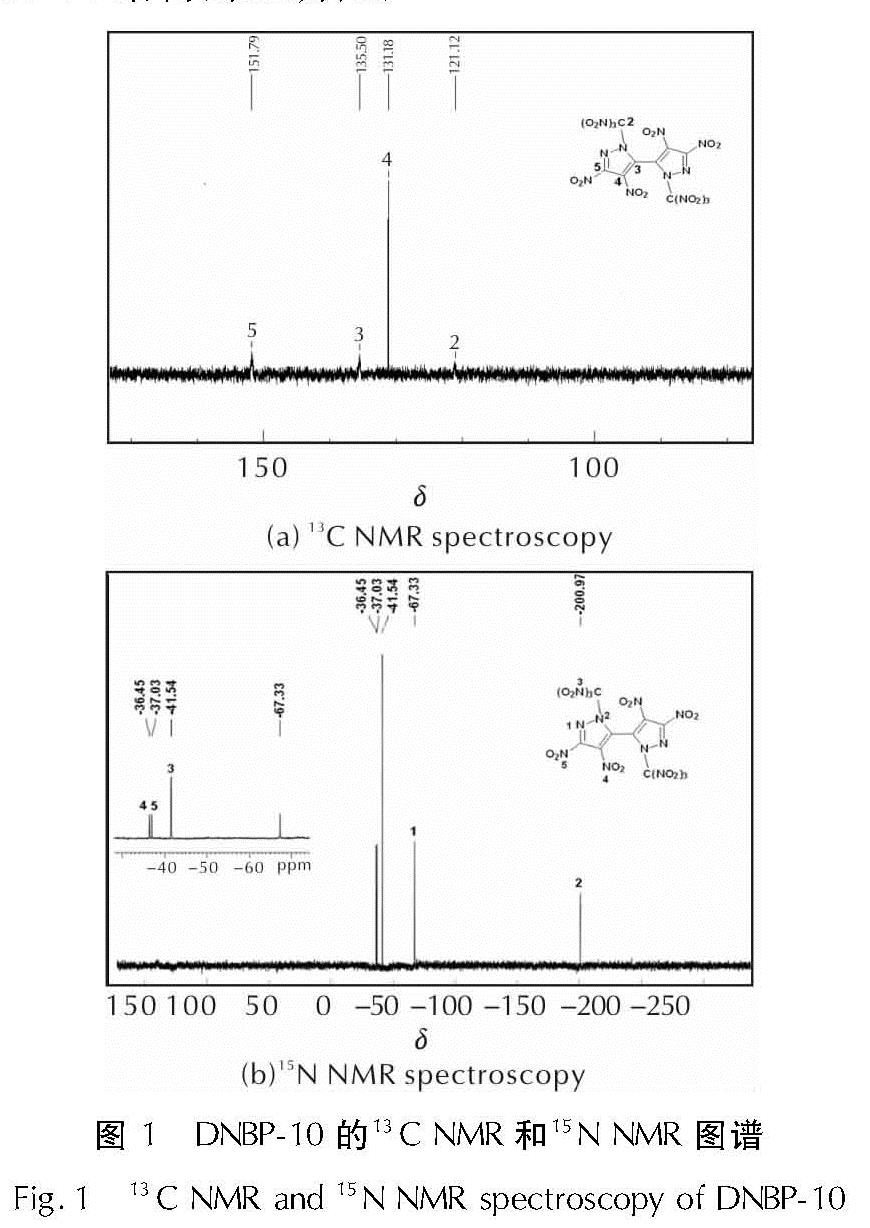
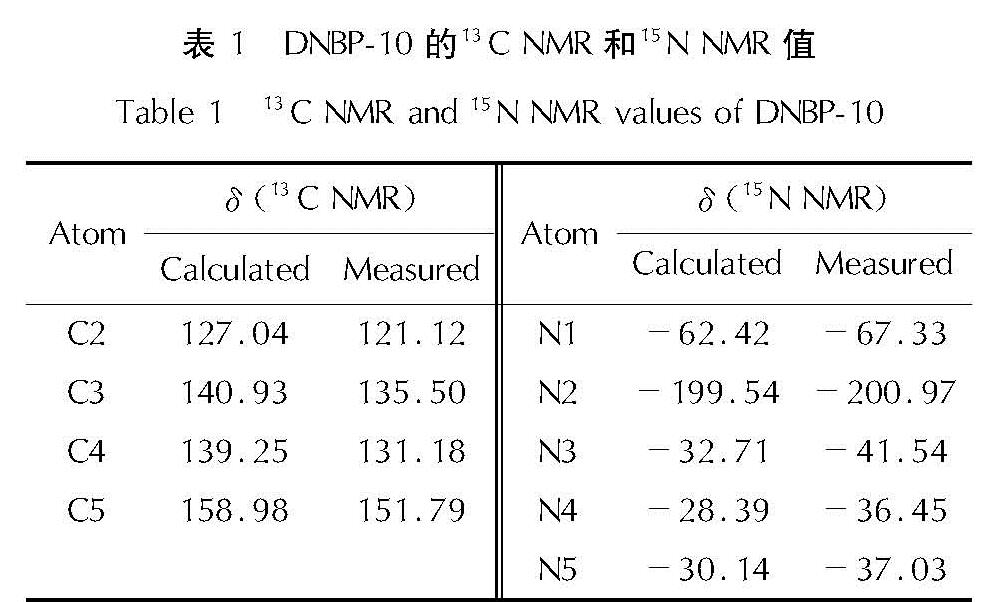
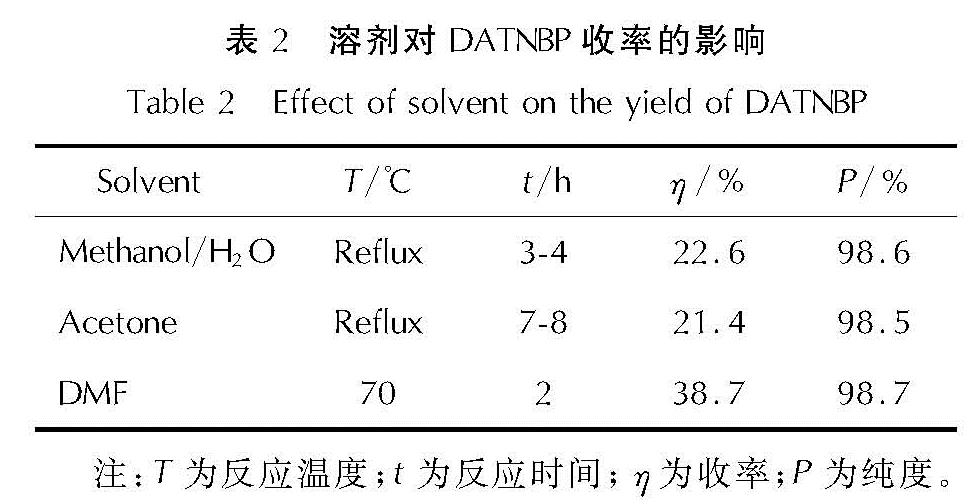
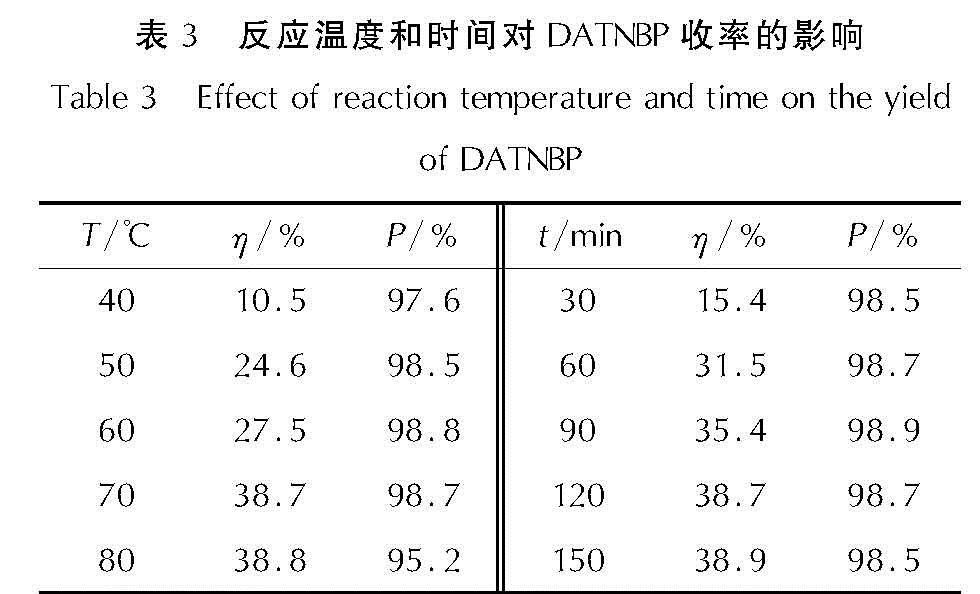
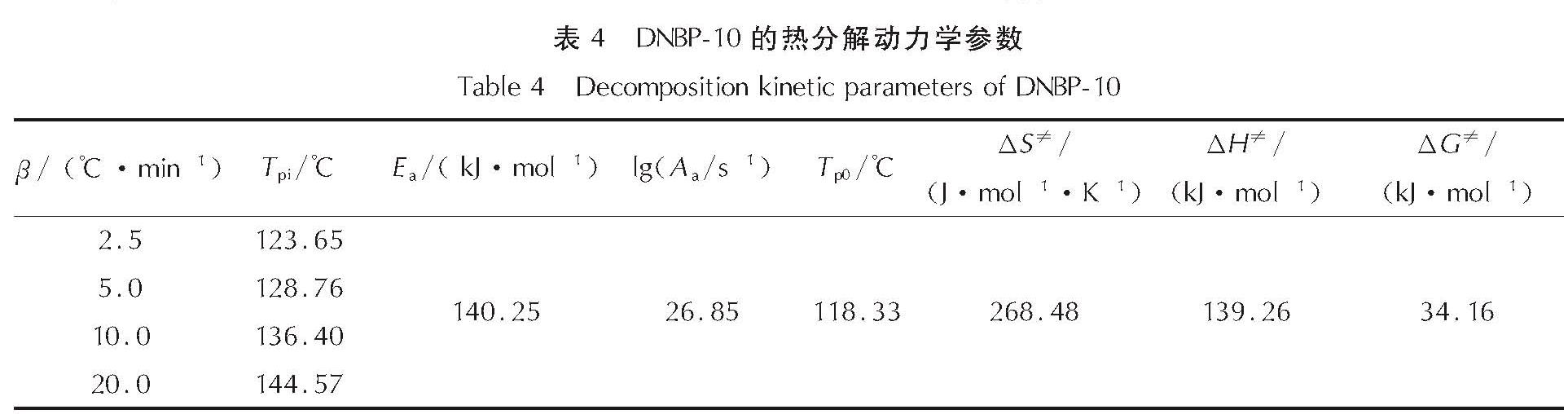
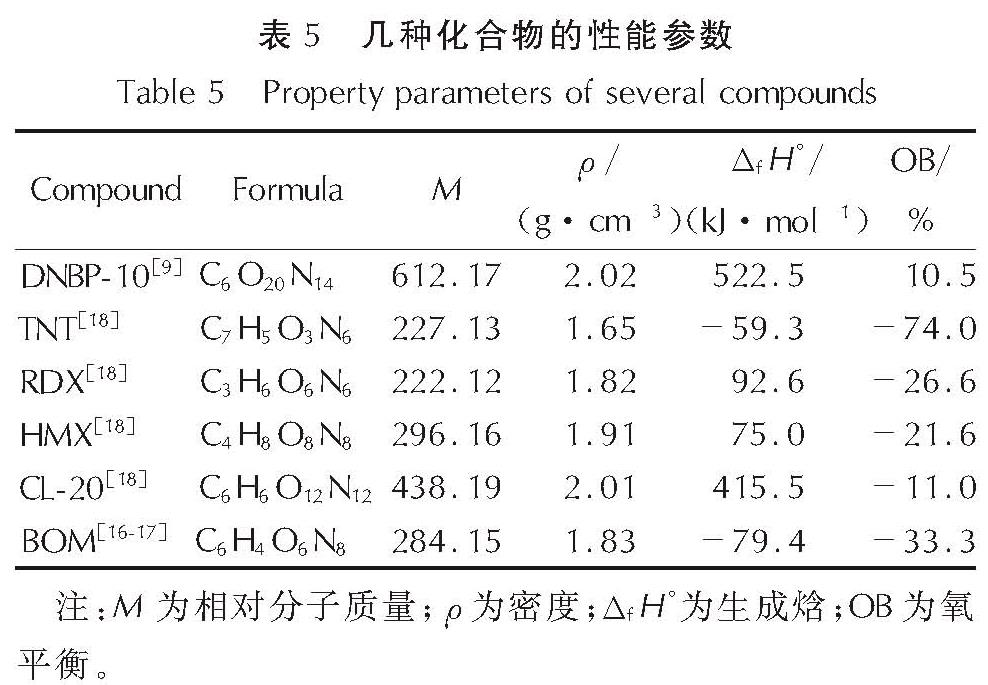
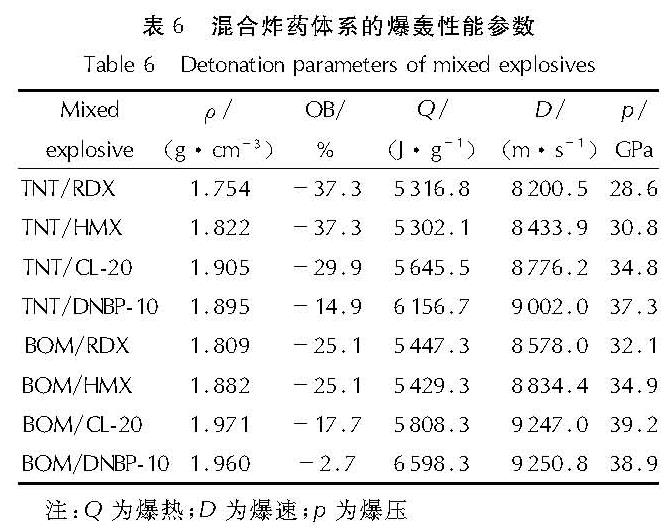
以4,4',5,5'-四硝基-2H,2'H-3,3'-联吡唑(TNBP)和氯丙酮为原料,经取代、硝化反应得到一种高能氧化剂4,4',5,5'-四硝基-2,2'-双(三硝甲基)-2H,2'H-3,3'-联吡唑(DNBP-10),总收率22.1%,并用红外光谱、13C NMR、15N NMR及元素分析等进行了结构表征; 对取代反应进行了工艺优化; 采用差示扫描量热和热重(DSC-TG)法研究了DNBP-10的热行为,并采用Kissinger方法计算了热分解参数; 采用EXPLO5软件对比分析了RDX、HMX、CL-20与DNBP-10对熔铸混合炸药爆轰性能的影响。结果 表明,氯丙酮和KBr作为取代试剂,在DMF中70℃反应2h得到取代产物,与文献反应时间72h对比,显著缩短了反应周期; DNBP-10的熔点和分解点分别为124.2和136.4℃; TNT/DNBP-10(30/70)和BOM/DNBP-10(30/70)的理论爆热分别为6156.7和6598.3J/g,比TNT/CL-20和BOM/CL-20分别高出511.2和790J/g,理论爆速分别达到9002.0和9250.8m/s,较TNT/CL-20提高225.8m/s,与BOM/CL-20相当,表明DNBP-10可大幅提高熔铸混合火炸药的能量性能。
A high energetic oxidizer, 4,4',5,5'-tetranitro-2,2'-bis(trinitromethyl)-2H,2'H-3,3'-bipyrazole(DNBP-10), was synthesized via substitution and nitration reaction with an overall yield of 22.1% using 4,4',5,5'-tetranitro-2H,2'H-3,3'-bipyrazole(TNBP)and chloroacetone as raw materials. Its structure was characterized by FT-IR spectra, 13C NMR, 15N NMR and elemental analysis. The reaction process of the substitution for the DNBP-10 was optimized. The thermal behaviors for DNBP-10 was studied by differential scanning calorimetry and thermogranvimetic(DSC-TG)techniques, and the thermal decomposition parameters were calculated by Kissinger method. The detonation parameters of TNT or BOM-based melt casting explosives with RDX, HMX, CL-20 and DNBP-10 as high energy phases were theoretically calculated by EXPLO5 software. The results show that the substitution condition is as follows: chloroacetone and KBr were regarded as the substitution regents, and the reaction time is 2h at 70℃ in DMF. The peak temperature of melting point and the exothermic decomposition reaction are 124.2℃ and 136.4℃, respectively. The theoretical heat of detonation of TNT/DNBP-10(30/70)and BOM/DNBP-10(30/70)are 6156.7 and 6598.3J/g, which are higher than those of TNT/CL-20(511.2J/g)and BOM/CL-20(790J/g), respectively. The theoretical detonation velocity for TNT/DNBP-10(30/70)and BOM/DNBP-10(30/70)are 9002.0 and 9250.8m/s, higher than TNT/CL-20(225.8m/s)and close to BOM/CL-20. Compared with RDX, HMX and CL-20, the energy is improved greatly with DNBP-10 as solid phase in melting and casting mixed explosives.