作者简介:郑婉婉(1995-),女,硕士研究生,从事含能材料合成研究。E-mail:1443924349@qq.com 通信作者:马海霞(1974-),女,教授,博导,从事含能材料合成、热力学性能及其量子化学研究。E-mail:mahx@nwu.edu.cn
(西北大学 化工学院,陕西 西安 710069)
(School of Chemical Engineering, Northwest University, Xi'an 710069, China)
organic chemistry; 1,2,4,5-tetrazine; crystal structure; nitrogen-rich energetic compounds; oxytetrazine derivatives; ketones
DOI: 10.14077/j.issn.1007-7812.202007029
备注
作者简介:郑婉婉(1995-),女,硕士研究生,从事含能材料合成研究。E-mail:1443924349@qq.com 通信作者:马海霞(1974-),女,教授,博导,从事含能材料合成、热力学性能及其量子化学研究。E-mail:mahx@nwu.edu.cn
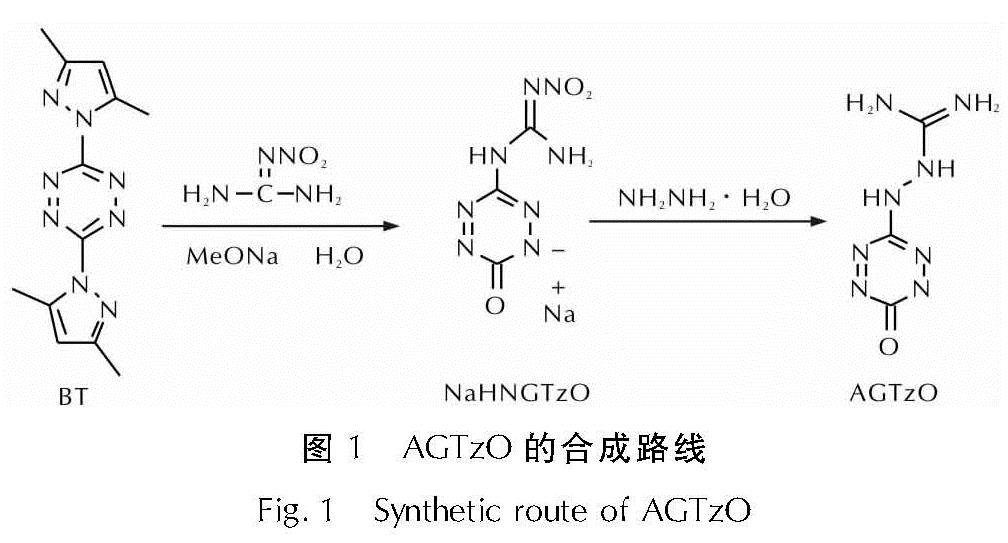
以3,6-双(3,5-二甲基吡唑基)-1,2,4,5-四嗪(BT)和硝基胍为原料,经亲核取代、还原反应,合成了6-氨基胍基-1,2,4,5-四嗪-3-酮(AGTzO),采用X-射线单晶衍射对AGTzO的结构进行表征; 利用差示扫描量热法(DSC)和热重法(TG-DTG)研究了AGTzO的热分解行为; 用Kissinger法和Ozawa法对不同升温速率下AGTzO的DSC曲线进行分析,获得热分解动力学参数,并分析了其热安全性。结果 表明,AGTzO·H2O的晶体属于正交晶系,Pbca空间群,每个晶胞包含8个AGTzO·H2O分子,晶体密度为 1.639g/cm3; AGTzO·H2O的活化能(E)和指前因子(A)分别为173.03kJ/mol、1015.96s-1; 自加速分解温度(TSADT)、热点火温度(Tbe)和热爆炸临界温度(Tbp)分别为232.05、234.93和236.91℃; 用氮当量公式计算AGTzO·H2O的爆速(D)为8421m/s,爆压(p)为30.4GPa,其爆轰性能与TATB(D=8114m/s,p=31.2GPa)相当。
Using 3,6-bis(3,5-dimethyllpyrazolyl)-1,2,4,5-tetrazine(BT)and nitroguanidine as raw materials, 6-aminoguanidine-1,2,4,5-tetrazine-3-one(AGTzO)was synthesized by the reactions of nucleophilic substitution and reduction. The structure of AGTzO was characterized by single crystal X-ray diffraction. The thermal decomposition behavior of AGTzO was analyzed by differential scanning calorimetry(DSC)and thermogravimetry(TG-DTG). The kinetic parameters were obtained by Kissinger and Ozawa methods using DSC curves at different heating rates,and the thermal safety was analyzed. The results show that the crystal of AGTzO·H2O belongs to the orthorhombic system, Pbca space group. Each unit cell contains eight AGTzO·H2O molecules, and the crystal density is 1.639g/cm3. The apparent activation energy(E)and pre-exponential constant(A)are 173.03 kJ/mol and 1015.96s-1, respectively. The self-accelerating decomposition temperature(TSADT), the thermal ignition temperature(Tbe)and the critical temperature of thermal explosion(Tbp)are 232.05, 234.93, and 236.91℃, respectively. The nitrogen equivalent formula was used to calculate the detonation velocity D(8421m/s)and the detonation pressure p(30.4GPa)of AGTzO·H2O. Its detonation performance is close to that of TATB(D=8114m/s,p=31.2GPa).
引言
富氮含能化合物在高能炸药、固体火箭推进剂、无烟烟火燃料和气体发生剂等领域有着重要的应用前景。为满足不断增长的性能要求,新的富氮含能材料日益向更高的密度、更好的爆轰性能、更低的冲击和摩擦灵敏度以及更高的热稳定性方向发展[1]。但由于含能化合物自身存在能量和稳定性之间的矛盾,多年来国内外研究者一直致力于研究含能性能和安全性之间的平衡[2]。四嗪类含能化合物因其具有较好的热稳定性、较低的感度以及较高的生成焓等优点备受含能材料研究者的青睐[3-7]。研究较多的是1,2,4,5-四嗪衍生物,其结构与苯环相似,符合休克尔(Hückel)规则,具有芳香性,虽然结构含有双键但是不容易发生亲电取代反应[8-9]。相较于苯环,四嗪环上N原子的引入增强了芳香性和碱性,而且N原子吸电子能力大于C原子,环上电子云密度向N转移,使得C上电子云密度减小,电负性减弱而成为亲核取代反应活性位点,易发生亲核取代反应。
2019年,张聪等[10]报道了6-(3,5-二甲基-1H-吡唑)-1,2,4,5-四嗪-3-酮(DPTzO)的合成,证明了四嗪酮类化合物结构的存在。为寻求性能良好的四嗪类含能化合物,本研究以 3,6-二(3,5-二甲基吡唑-1-基)-1,2,4,5-四嗪(BT)与硝基胍为原料,经亲核取代、还原反应,合成了6-氨基胍基-1,2,4,5-四嗪-3-酮(AGTzO),并确定了AGTzO的晶体结构; 研究了AGTzO的热分解行为,获得热分解动力学参数,并初步评价其热安全性; 计算了AGTzO的爆速和爆压。研究表明AGTzO具有良好的热稳定性,AGTzO中酮式结构的存在增加了分子的氧含量,使分子更加容易达到氧平衡,有望提高四嗪类含能化合物的密度和爆轰性能,为含氧四嗪衍生物在含能材料领域的应用提供理论依据。
1 实 验
1.1 试剂与仪器硝基胍,分析纯,上海阿拉丁试剂有限公司; 质量分数80%水合肼、甲醇钠,均为分析纯,国药集团化学试剂有限公司; 3,6-双(3,5-二甲基吡唑)-1,2,4,5-四嗪(BT),自制。
DFY-5L/40低温反应浴槽,郑州予华仪器公司; Vario EL Cube型元素分析仪,德国Element仪器公司; IRAffinity-1S型傅里叶红外光谱仪,日本Shimadzu公司; DSC-Q2000型差示扫描量热仪、TGA/SDT-Q600型热分析仪,美国TA公司; SMART APEX CCD 型单晶衍射仪,德国Bruker公司。
1.2 AGTzO的制备AGTzO的合成路线如图 1所示,其中3,6-双(3,5-二甲基吡唑基)-1,2,4,5-四嗪(BT)
参考文献[11-12]合成。
将硝基胍(1.04g,10mmol),甲醇钠(6.5mL,30mmol)溶液加入三口烧瓶中,升温至50℃同时剧烈搅拌,之后依次加入去离子水(1mL,60mmol)和 BT(2.7g,10mmol),5h后停止反应。趁热过滤,得到1.429g NaHNGTzO的红色固体,产率为64.4%。
将NaHNGTzO(0.222g,1mmol)溶于10mL水,置于低温浴槽中,逐滴滴加0.5mL、质量分数80%水合肼,室温下搅拌30min,过滤,滤液静置一段时间后有0.098g AGTzO的深红色固体析出,产率为52.4%。
AGTzO·H2O,元素分析(C3H8N8O2,%):计算值,C 19.14,H 4.26,N 59.57; 实测值,C 19.03,H 4.45,N 58.98。IR(KBr),ν(cm-1):3377,3139,2865,2368,1684,1647,1438,1322,1279,1208,1063,955,825,753,617。
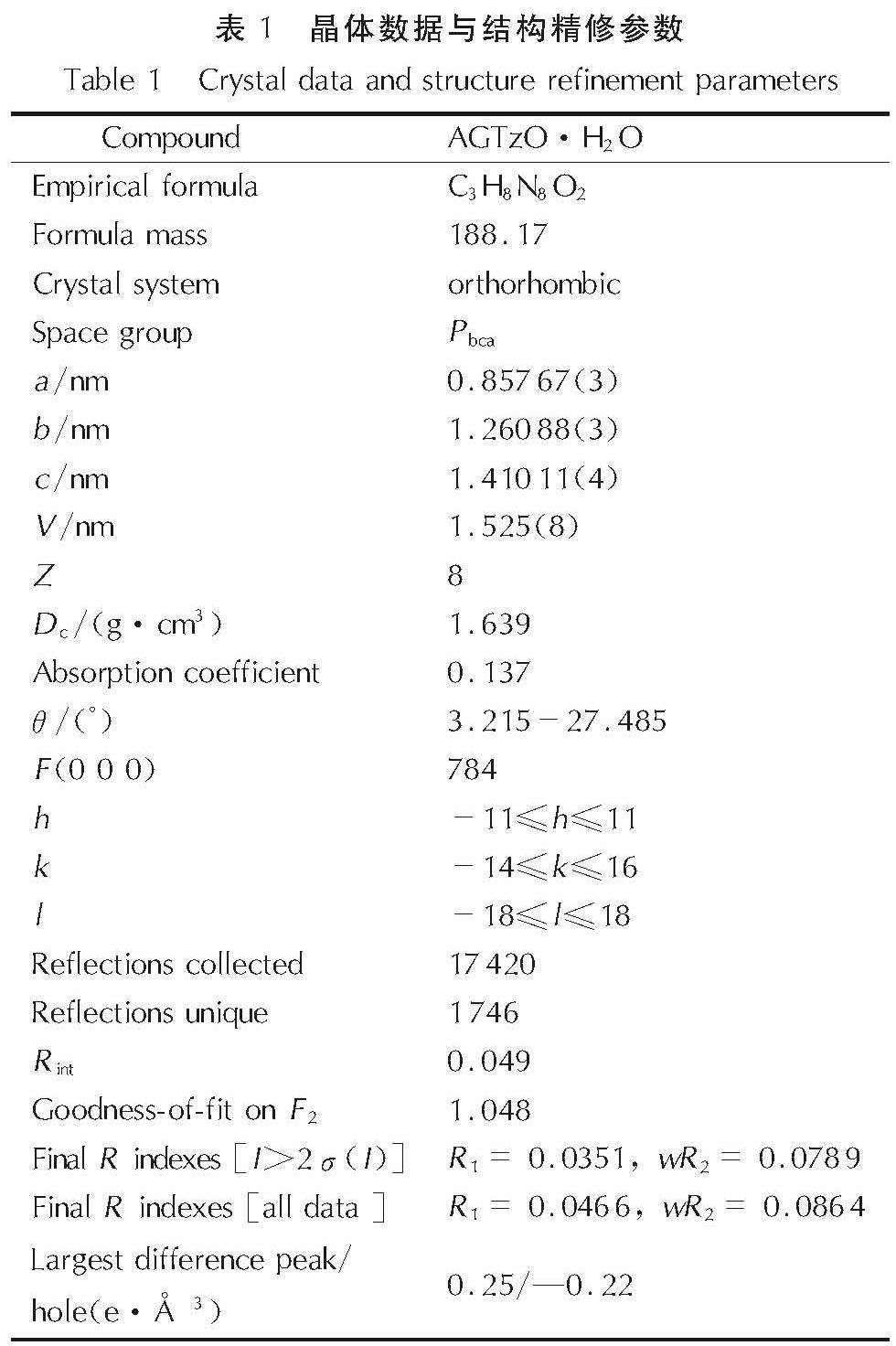
1.3 单晶培养和测试将AGTzO固体溶于去离子水中制成饱和溶液,用溶剂挥发法培养单晶,5天后得到可用于结构测定的深红色针状晶体。选取尺寸为0.12mm×0.12mm×0.03mm透明有光泽、形状规整的晶体,以φ-ω扫描方式,用波长为0.071073nm的Mo Kα射线,在296(2)K下进行扫描和多次扫描吸收校正。分子结构用SHELXS-97程序由直接法求得,在SHELXL-97[13-14]程序中用基于F2的全矩阵最小二乘法进行精修。晶体学参数列于表1,该晶体的CCDC号为19806397。
1.4 热分析测试DSC测试:样品质量为0.1~0.3mg,升温速率为5、10、15、20℃/min,N2流速为50mL/min。
TG-DTG测试:样品质量约为0.4mg,升温速率为10℃/min,N2流速为100mL/min。
2 结果与讨论
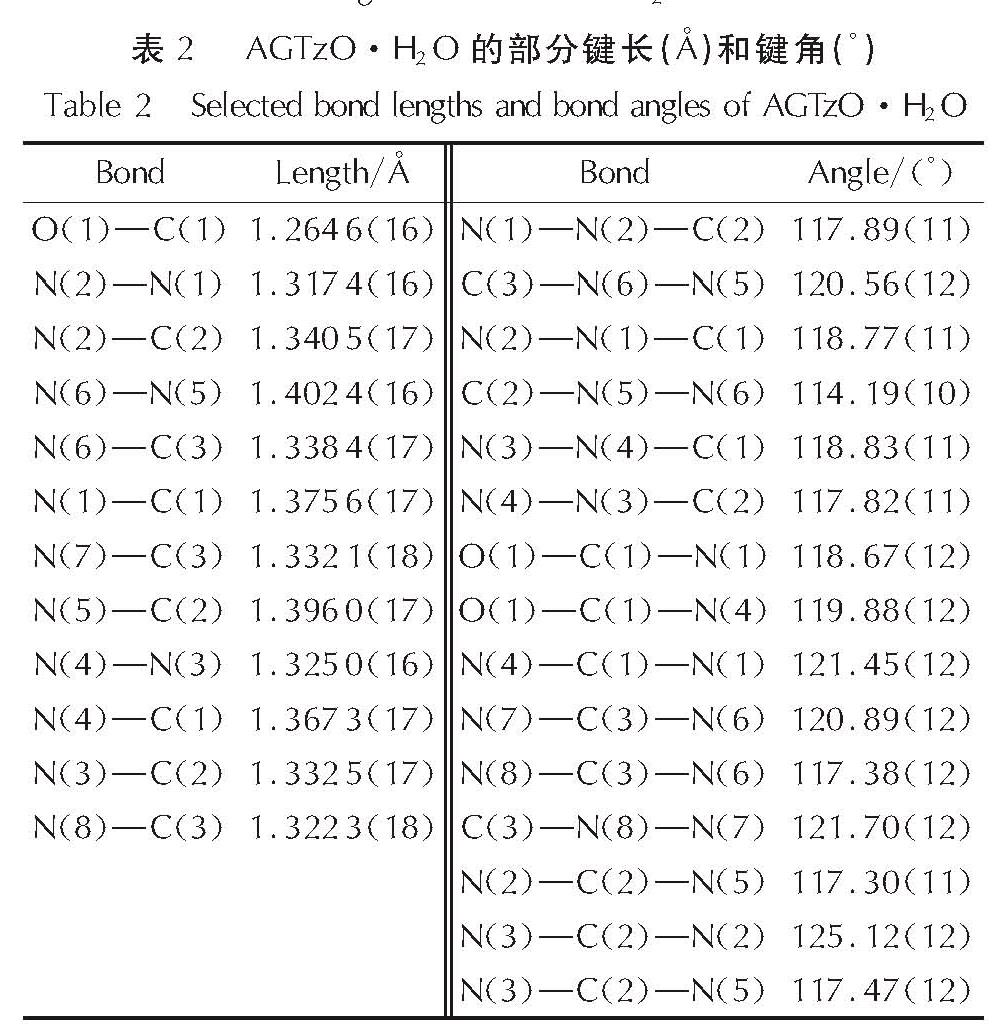
2.1 晶体结构分析AGTzO·H2O的分子结构图、氢键图和堆积图如图2所示,部分键长、键角和氢键数据列于表2和表3。晶体结构分析表明:AGTzO·H2O是正交晶系,空间群为Pbca,每个结构单元内包含8个分子。由图2(a)可知,O(1)和C(1)直接相连,O(1)上没有H原子,表明与四嗪环相连的氧原子以酮式结构存在。由表2可知,C(2)─N(2)(1.34Å)和C(2)─N(5)(1.396Å)键长介于C─N(1.47Å)和C=N(1.22Å)之间,表明该分子内存在大的π共轭结构,导致高程度的电子离域[15],从而增加环的稳定性。AGTzO分子内原子通过分子内氢键N(7)─H(7B)…N(5)(v)连接,形成稳定的五元环结构,如图2(b)所示,氢键的键长及键角如表3所示。同时,AGTzO和另一个AGTzO及水分子之间存在大量的分子间氢键,且一个AGTzO分子与相邻的一个AGTzO通过N(7)─H(7A)…N(3)(iv)和N(8)─H(8A)…N(3)(iv) 连接,形成稳定的六元环状结构,与相邻的另一个AGTzO分子通过N(6)─H(6)…O(1)(iii)与N(8)─H(8B)…N(1)(iii)连接,形成一个八元环状结构。AGTzO分子与水分子通过分子间氢键O(2)─H(2A)…N(2)i与O(2)─H(2B)…O(1)连接,形成二维平面结构,二维平面结构在范德华力作用下形成一个紧密排列的波浪状堆积结构,如图2(c)所示。
图2 AGTzO·H2O的分子结构、氢键和晶胞堆积图
Fig.2 Molecular structure, hydrogen-bonds and packing diagram of AGTzO·H2O表2 AGTzO·H2O的部分键长(Å)和键角(°)
Table 2 Selected bond lengths and bond angles of AGTzO·H2O表3 AGTzO·H2O氢键的键长(Å)和键角(°)
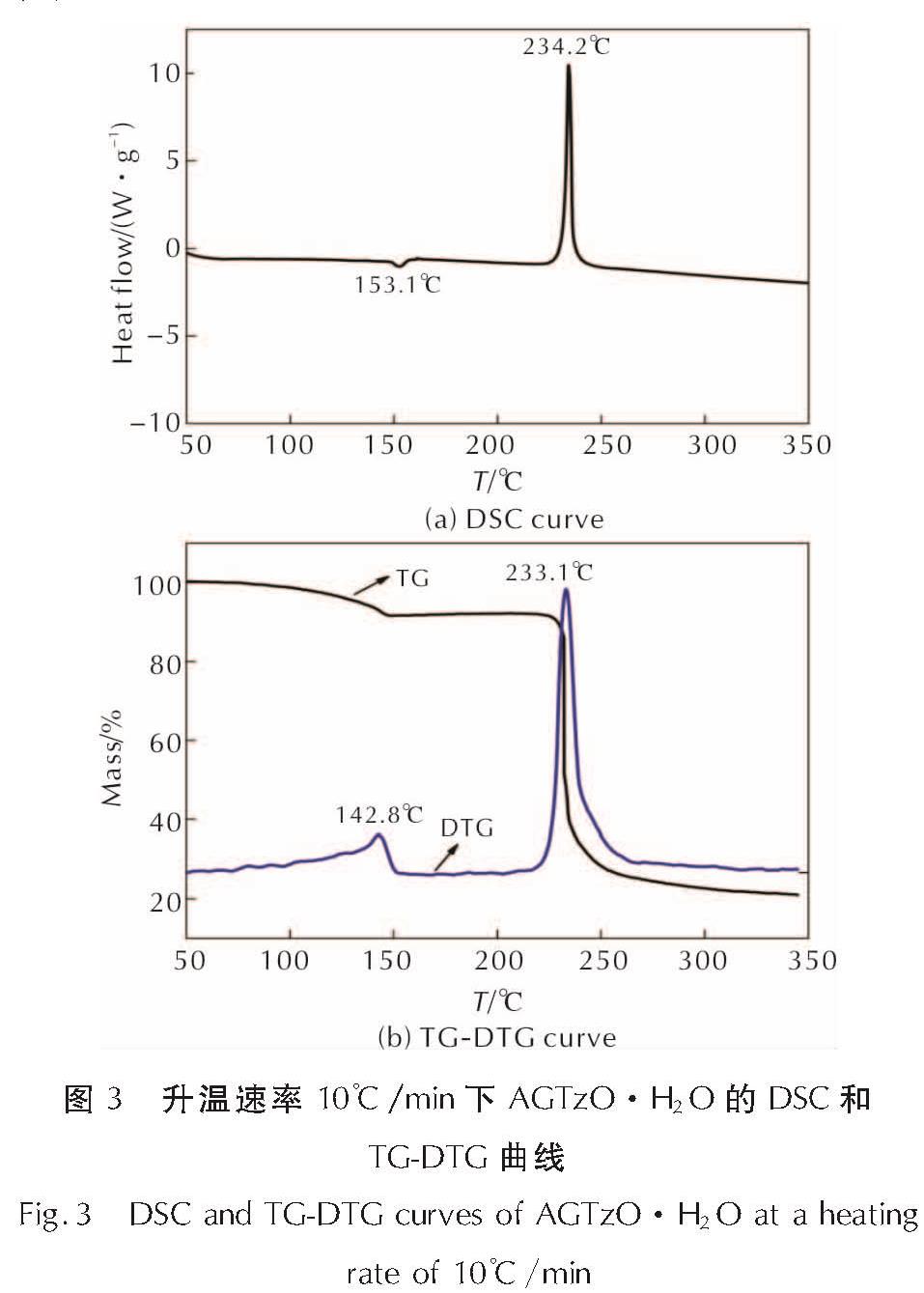
Table 3 Hydrogen bond lengths (Å) and angles (°) for AGTzO·H2O2.2 热分解行为与热分解反应动力学AGTzO·H2O的DSC和TG-DTG曲线如图3所示。
图3 升温速率10℃/min下AGTzO·H2O的DSC和TG-DTG曲线
Fig.3 DSC and TG-DTG curves of AGTzO·H2O at a heating rate of 10℃/min由图3(a)可以看出,AGTzO·H2O有1个吸热过程,吸热峰温为153.1℃,对应于AGTzO·H2O失去结晶水所产生的吸热效应,对应于图3(b)TG曲线上第一个失重过程,质量损失为8.34%,与失去分子中的一个结合水9.57%基本一致。AGTzO·H2O有一个快速放热阶段,峰温为234.2℃,外推起始温度为232.1℃,放热量Q为1397J/g,对应于图3(b)AGTzO·H2O的快速失重阶段,始于215.7℃,终于264.9℃,失重率为62.76%,失重速率最大时对应温度为233.1℃,与DSC曲线上快速放热阶段峰温值接近。
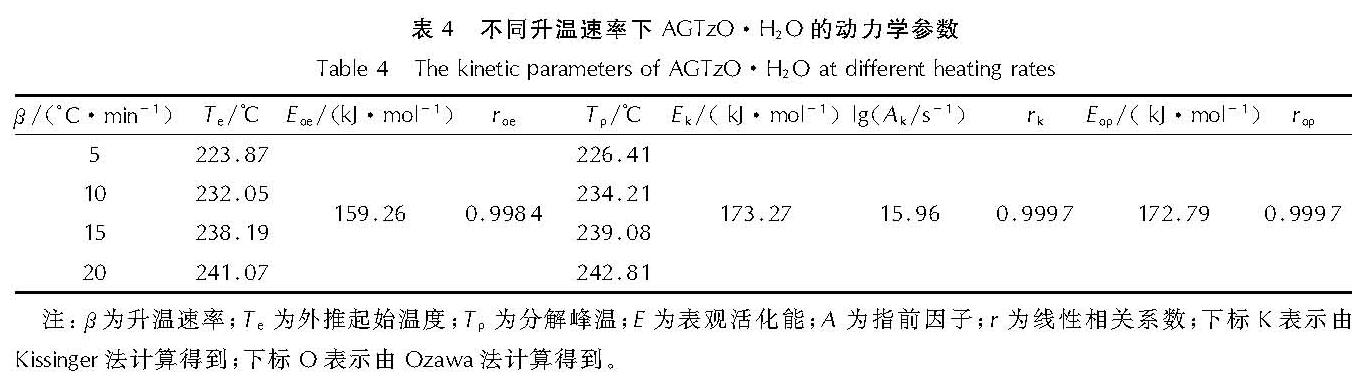
表4为在不同升温速率(β)下的DSC曲线放热峰得到的动力学参数及由Kissinger[16]方程和Ozawa[17]方程计算的热分解反应的表观活化能(E)。由计算结果可知,两种方法计算的E基本接近且相关性大于0.98。说明在整个分解温度范围内热分解所遵循的机理函数一致。
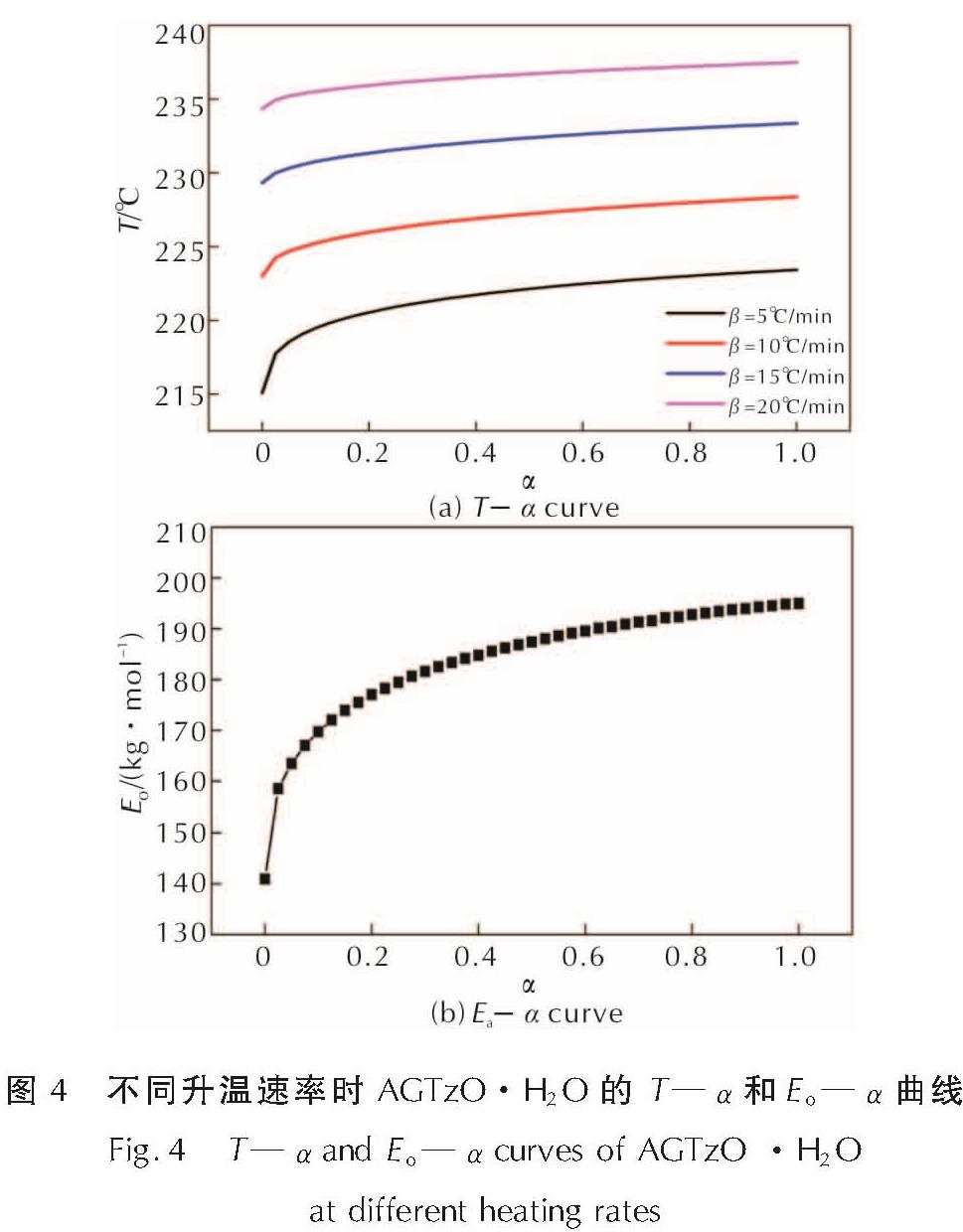
将升温速率(βi)、反应分数(αi)和反应温度(Ti)(i=1,2,…)代入Ozawa方程中,得到了表观活化能Eo。AGTzO·H2O的T—α、Eo—α曲线关系如图4所示,发现不同升温速率下的温度变化曲线相同。当α在0.65~0.875之间时,Eo值几乎趋于水平,介于190.40 ~ 193.77kJ/mol之间,Eo值相近,因此选取α为0.65 ~ 0.875研究AGTzO·H2O的非等温热分解反应动力学。
图4 不同升温速率时AGTzO·H2O的T—α和Eo—α曲线
Fig.4 T—α and Eo—α curves of AGTzO ·H2O at different heating rates将DSC热分解数据T—α代入到41种机理函数[18]进行线性回归计算,求出General Integral、Universal integral、MacCallum-Tanner、Satava-Sesta、Agrawal方程(公式(1)~(5))所对应的表观活化能E、指前因子A、线性相关系数r和标准方差Q[19-20]。
表4 不同升温速率下AGTzO·H2O的动力学参数
Table 4 The kinetic parameters of AGTzO·H2O at different heating ratesGeneral Integra方程:

Satava-Sesta方程:
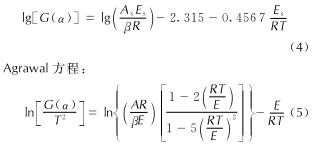
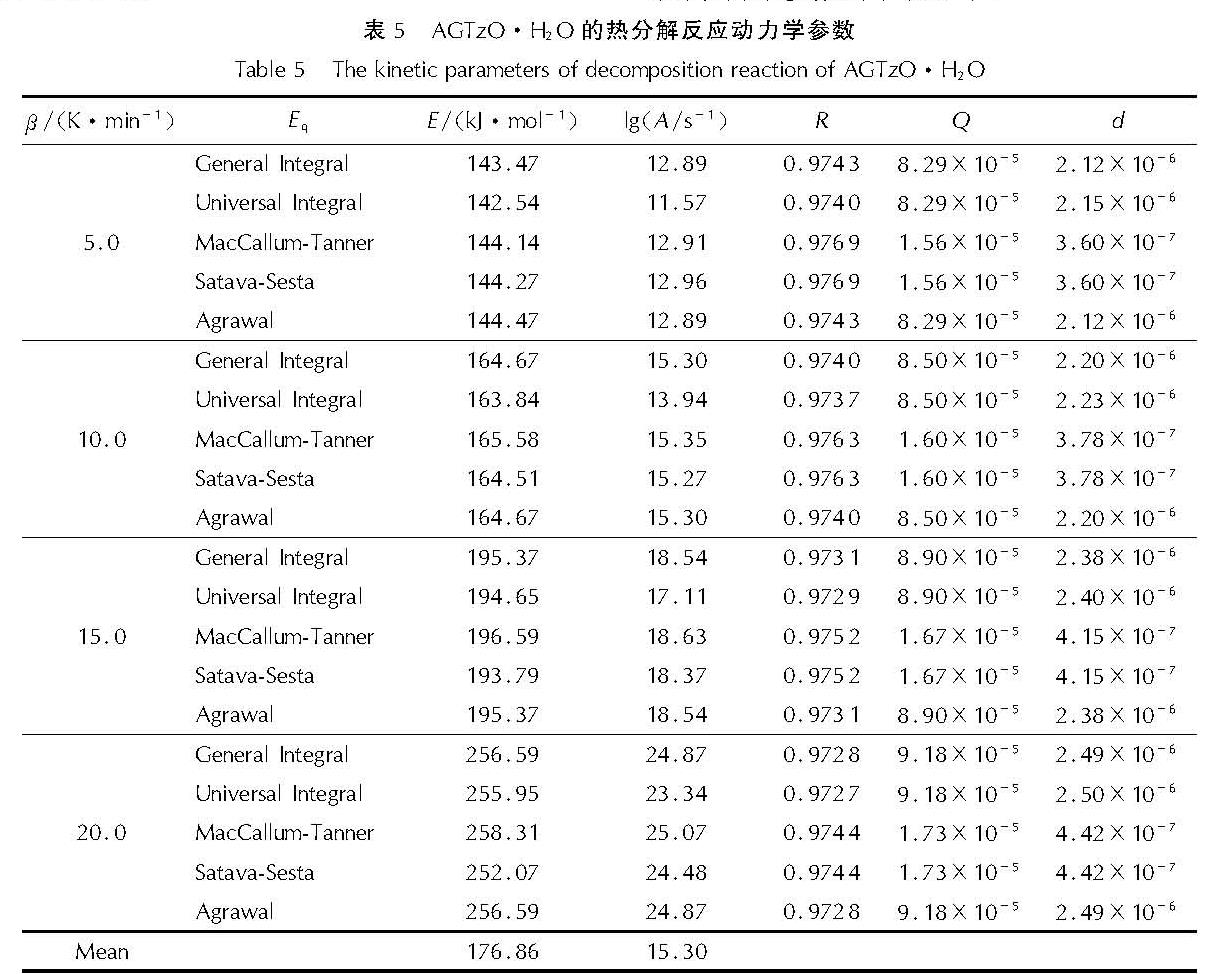
对比不同方法所求得的动力学参数,结果表明AGTzO·H2O热分解反应的动力学符合第34号函数:f(α)=(1-α)-2/3,并将第34号机理函数得到的动力学参数列于表5中。
表5 AGTzO·H2O的热分解反应动力学参数
Table 5 The kinetic parameters of decomposition reaction of AGTzO·H2O表5中数据表明,升温速率为5、10、15℃/min下的E和A值分布相对均匀,而20℃/min的升温速率下的E、A存在较大的偏差,因此通过计算求得5、10、15℃/min下的E和A的平均值分别为176.86kJ/mol和1015.30s-1。将E=176.86kJ/mol,A=1015.30s-1和f(α)代入方程(6):
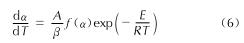
得到AGTzO·H2O热分解反应动力学方程:
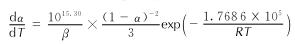
自加速分解温度(TSADT)、热点火温度(Tbe)和热爆炸临界温度(Tbp)是评价含能材料的热安全性重要参数。联合方程(7)、(8)、(9)可以得到化合物的TSADT、Tbe和Tbp[21-22]。其中外推起始分解温度(Te0)与外推峰顶温度(Tp0)是指方程(7)在升温速率β→0时的取值。
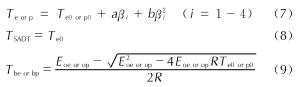
表6 AGTzO·H2O热分解过程中的温度参数
Table 6 Temperature parameters in thermal decomposition of AGTzO·H2O由表6可知,AGTzO·H2O的TSADT、Tbe和Tbp分别为:232.05、234.93和236.91℃,均高于200℃,可能因为AGTzO·H2O存在大量分子内和分子间氢键,从而提高了自身的热稳定性。
2.3 爆速和爆压的计算爆速(D)和爆压(p)的计算采用氮当量公式[23-25],其公式如下:
∑N=100∑xiNi/M(10)
D=(690+1160ρ0)∑N(11)
ρ=1.092(ρ0∑N)2-0.574(12)
式中:∑N为含能材料的氮当量; xi为每分子含能材料爆轰时生成的某种产物的分子数; Ni为爆轰产物的氮当量系数; ρ0为材料的密度(g/cm3)。
含能材料爆轰产物的氮当量系数分别为:N2 1、H2O 0.54、CO 0.78、CO2 1.35、C 0.15、H2 0.29。AGTzO·H2O的爆轰产物见式(13),据此计算获得其∑N为3.25,相应的爆速(D)和爆压(p)分别为8421m/s和30.4GPa,与TATB(D=8114m/s,p=31.2GPa)相当。可能是由于AGTzO·H2O 的分子内存在氧原子,提高了其密度和氧平衡,从而提高其爆轰性能。
C3H8N8O2=2H2O+2H2+3C+4N2(13)
3 结 论
(1)合成出AGTzO并获得单晶, AGTzO·H2O属于正交晶系,Pbca空间群,每个晶胞包含8个AGTzO·H2O分子,密度为1.639g/cm3。
(2)AGTzO·H2O有一个快速放热阶段, AGTzO·H2O 的活化能(E)和指前因子(A)分别为173.03kJ/mol和1015.96s-1; 自加速分解温度(TSADT)、热点火温度(Tbe)以及热爆炸临界温度(Tbp)分别为232.05、234.93、236.91。
(3)AGTzO·H2O 的晶体结构中含大量氢键,因此其热稳定性较优。AGTzO·H2O的爆速(D)为8421m/s,爆压(p)为30.4GPa,故以化合物AGTzO为基础有望合成出具有广阔应用前景的新型含能材料。
- [1] CHAVEZ D E, HISKEY M A. 1,2,4,5-Tetrazine based energetic materials [J]. Journal of Energetic Materials, 1999,17(4):357-377.
- [2]阳世清,岳守体. 国外四嗪四唑类高氮含能材料研究进展[J]. 含能材料, 2003,11(4):231-235.
- [3]何冬梅,程广斌,吕春绪. 四嗪类高氮含能化合物的合成与表征[J]. 火炸药学报,2010, 33(5):8-11.
- [4]曾天,韩雪,陈湘,等. 不对称1,2,4,5-四嗪类化合物DPHX和DMHT的结构、热行为和热安全性[J]. 含能材料,2018, 26(10):856-863.
- [5]CHEN Dan, YANG Hong-wei, YI Zhen-xin, et al. C8N26H4: an environmentally friendly primary explosive with high heat of formation [J]. Angewandte Chemie International Edition,2018,57(10):2081-2084.
- [6]张海昊,贾思媛,王伯周,等. 3,6-二肼基-1,2,4,5-四嗪及其含能盐的合成与性能[J].火炸药学报, 2014.37(2):23-26.
- [7]STEELE B A, STAVROUS E,CROWHURST J C,et al. High-pressure synthesis of a pentazolate salt [J]. Chemistry of Materials,2017,29(2):735-741.
- [8]PENG Feng, YAO Yan-sun, LIU Han-yu, et al. Crystalline LiN5 predicted from first-principles as a possible high-energy material [J]. Journal of Physical Chemistry Letters,2015,6(12):2363-2366.
- [9]XU Yuan-gang, LIN Qiu-han, WANG Peng-cheng, et al. Syntheses, crystal structures and properties of a series of 3D metal-inorganic frameworks containing pentazolate anion[J]. Chemistry An Asian Journal,2018.DOI:10.1002/asia.201800476
- [10]张聪,陈湘,白杨,等. 6-(3,5-二甲基-1H-吡唑)-1,2,4,5-四嗪-3-酮(DPTzO)及其胍盐的晶体结构和热分解行为[J]. 火炸药学报, 2019, 42(5): 432-444.
- [11]胡拥鹏. 均四嗪类含能物的合成、结构、热行为及安全性研究[D].西安:西北大学,2015.
- [12]李帅磊. 不对称 1,2,4,5-四嗪类含能材料的合成、结构及热行为研究[D].西安:西北大学,2016.
- [13]SHELDRICK G M. SHELXTL-97, Structure program for crystal structure solution[CP]. Göttingen:University of Göttingen,1997.
- [14]SHELDRICK G M. SHELXTL-97, Structure program for crystal structure refinement[CP]. Göttingen:University of Göttingen, 1997.
- [15]ZHANG Jia-heng, ZHANG Qing-hua, VO T T, et al. Energetic salts with π-stacking and hydrogen-bonding interactions lead the way to future energetic materials [J]. Journal of the American Chemical Society, 2015, 137(4): 1697-704.
- [16]KISSINGER H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29(11):1702-1706.
- [17]OZAWA T B. A new method of analyzing thermogravimatric data [J]. Bulletin of the Chemical Society of Japan, 1965, 38(11): 1881-1886.
- [18]胡荣祖,史启祯. 热分析动力学[M].北京:科学出版社,2001:15-18.
- [19]YAN Biao, LI Hong-ya, ZHAO Ning-ning, et al. Thermodynamic properties and detonation characterization of 3,3-dinitroazetidinium hydrochloride[J]. Journal of Chemical and Engineering Data,2013, 58(11): 3033-3038.
- [20]MA Hai-xia, YAN Biao, REN Ying-hui, et al. Thermal behavior and thermal safety on 3,3-dinitroazetidinium salt of perchloric acid[J]. Journal of Thermal Analysis and Calorimetry, 2011, 103(2):569-575.
- [21]MA Hai-xia, YAN Biao, LI Jun-feng, et al. Molecular structure, thermal behavior and adiabatic time-to-explosion of 3,3-dinitroazetidinium picrate[J]. Journal of Molecular Structure, 2010, 981(1-3): 103-110.
- [22]MA Hai-xia, YAN Biao, LI Zhao-na, et al. Preparation, non-isothermal decomposition kinetics, heat capacity and adiabatic time-to-explosion of NTO·DNAZ[J]. Journal of Hazardous Materials, 2009, 169(1-3): 1068-1073.
- [23]CHEN Hua-xiong, CHEN Sun-shen, LI Li-jie, et al. Synthesis, single crystal structure and characterization of pentanitromonoformyl hexaazaisowurtzitane [J]. Journal of Hazardous Materials, 2010, 175(1-3):569-574.
- [24]国迂贤, 张厚生. 炸药爆轰性质计算的氮当量公式及修正氮当量公式:炸药爆速的计算[J]. 爆炸与冲击, 1983, 3(3): 56-66.
- [25]张厚生. 用氮当量公式及修正氮当量公式计算炸药爆轰压力[J]. 爆炸与冲击, 1984, 4(2): 79-82.