作者简介:武碧栋(1985-),男,副教授,从事含能材料制备研究。E-mail:wubidong@nuc.edu.cn
(1.中北大学 环境与安全工程学院,山西 太原 030051; 2.山西省超细粉体工程技术研究中心,山西 太原 030051)
(1.School of Environment and Safety Engineering, North University of China, Taiyuan 030051,China; 2.Shanxi Engineering Technology Research Center for Ultrafine Powder, Taiyuan 030051,China)
organic chemistry; cocrystal; 3-amino-4-(tetrazol-5-yl)-furazan; HAFT; EDN; crystal structure; thermal analysis
DOI: 10.14077/j.issn.1007-7812.202004015
备注
作者简介:武碧栋(1985-),男,副教授,从事含能材料制备研究。E-mail:wubidong@nuc.edu.cn
以3-氨基-4-(四唑-5-基)呋咱(HAFT)和硝酸乙二胺(EDN)为原料,制备得到二元共晶HAFT·EDN,采用X射线衍射仪(XRD)、红外光谱、元素分析和X-射线单晶衍射仪表征了目标物结构; 采用差示扫描量热(DSC)对HAFT·EDN共晶进行了热性能测试,并测试了其撞击感度和摩擦感度; 通过Kissinger法、Ozawa法和Starink法计算分析了非等温动力学、热爆炸临界温度等性能参数。 结果 表明,该晶体属于单斜晶系,空间群为P21/c,晶体学参数为:a=7.515(4)Å,b=9.823(5)Å,c=14.828(8)Å,β=86.152(9)Å,V=1092.1(10)Å3,Dc=1.680g/cm3,其活化能为127.58kJ/mol,热爆炸临界温度为283.7℃,分解反应过程活化熵(ΔS≠)为-44.08kJ/mol,活化焓(ΔH≠)为119.68kJ/mol,吉布斯自由能(ΔG≠)为143.33kJ/mol,具有较好的稳定性,对撞击和摩擦刺激均钝感。
By using 3-amino-4-(tetrazol-5-yl)furazan(HAFT)and ethylenediaminenitrate(EDN)as raw materials, a binary cocrystal HAFT·EDN was prepared. X-ray diffractometry(XRD), infrared spectroscopy, elemental analysis and single crystal X-ray diffractometry were used to characterize the structure. The thermal properties of HAFT·EDN cocrystal were tested by differential scanning calorimetry(DSC). The impact and friction sensitivities were also tested.The Kissinger method, Ozawa method and Starink method were used to calculate and analyze the non-isothermal kinetic parameters and thermal explosion critical temperature.The results show that HAFT·EDN cocrystal belongs to monoclinic system with P21/c space group, crystallographic parameters: a=7.515(4)Å, b=9.823(5)Å, c=14.828(8)Å, β=86.152(9)Å, V=1092.1(10)Å3 and Dc=1.680g/cm3, where HAFT is presented as electrically neutral state. The activation energy is 127.58kJ/mol, and the thermal explosion critical temperature is 283.7℃. The activation entropy(ΔS≠)of the decomposition reaction process is -44.08kJ/mol, the activation enthalpy(ΔH≠)is119.68kJ/mol, and the Gibbs free energy(ΔG≠)is 143.33kJ/mol, showing good thermal stability and low sensitivity towards impact and friction stimuli.
引言
共晶是由两种或两种以上的物质在分子间氢键或其他非共价键作用下,以固定的化学计量比结合在同一晶格中,形成独特的多组分分子晶体。共晶是一种从分子结构上改善炸药性能的技术,可以有效降低炸药感度,优化炸药能量输出结构,改善其力学性能、表界面性能以及热稳定性等[1-7]。
唑类化合物具有高氮含量、高生成焓、高产气量和爆轰产物清洁等特点,唑环的生成焓随氮含量的增加而逐步增高,但稳定性随之降低,而四唑的氮含量适中,同时保有了较高的生成焓和稳定性。在含能材料领域具有广泛的应用[8]。俄罗斯科学院Zelinsky有机化学研究所对呋咱类含能化合物进行的20多年研究表明[9],对于含C、H、N、O原子的高能量密度化合物,呋咱环是一个非常有效的结构单元。呋咱(氧化呋咱)具有高密度、高的正生成热和良好的氧平衡,在改善炸药性能方面有重要意义[10-11]。2015年于倩倩等[12]基于3-硝基-4-(四唑-5-基)呋咱合成了3种含能离子盐,其中呋咱羟胺盐的能量最高,略高于RDX,是一种具有潜力的富氮含能材料; 2016年章雷[13]合成了3-硝基-4-(四唑基)呋咱铵盐,并对3-氨基-4-(1H-5四唑)呋咱和中间体1-氨基-2-氰基二肟的合成方法进行工艺改良,产物后处理简单,对环境较为友好; 2017年梁洁[14]合成了5种四唑基呋咱类含能化合物,均表现出较低的感度; 2019年丁自美[15]基于3-氨基-4-(四唑-5-基)呋咱(HAFT)合成了一系列含能配合物,其性能良好,另外HAFT所带的端氨基基团也比较活泼,为HAFT的研究提供了新思路。因此,具有呋咱环与四唑环相连的HAFT化合物,既能满足对能量的要求,又能保证其结构的稳定性。综上所述,对其开展的研究工作主要为:将HAFT的氨基进行硝基化取代形成高能硝基化合物; HAFT中四唑基脱去氢原子形成高氮阴离子,与高氮阳离子结合形成高氮离子盐; 与过渡金属进行配位反应形成含能配合物,用于燃速催化剂或起爆药。
HAFT作为中性化合物在共晶方面的研究还未见报道。本研究通过溶液滴加法将3-氨基-4-(四唑-5-基)呋咱、乙二胺和硝酸的离子盐合成二元共晶HAFT·EDN,培养了目标物的单晶并对其结构进行了分析,并研究了其热稳定性能,为进一步开展中性炸药分子和离子型炸药形成共晶的研究提供参考。
1 实 验
1.1 试剂与仪器3-氨基-4-(四唑-5-基)呋咱,根据文献[16-22]自制; 乙二胺(质量分数≥99%),分析纯,天津天力化学试剂有限公司; 浓硝酸(质量分数65%~68%),西陇化工股份有限公司。
RE-2000E型旋转蒸发器,西安仪贝尔仪器设备有限公司; SHZ-D(Ⅲ)型循环水式多用真空泵,上海力辰邦西仪器科技有限公司; VARI-EL-3/0型元素分析仪,德国Exementar公司; SMART APEX CCD型单晶衍射仪,德国Bruker公司; DX-2700型X射线衍射仪,丹东浩元仪器有限公司; FTIR-7600型红外光谱仪,天津分析仪器厂; DSC-800型差示扫描量热仪,上海皆淮仪器设备有限公司; ERL-12撞击感度仪、WM-1摩擦感度仪,中国兵器工业传爆药性能检测中心。
1.2 HAFT·EDN共晶的合成将1.53g(10mmol)3-氨基-4-(四唑-5-基)呋咱和25mL蒸馏水加入三口烧瓶,搅拌速率250r/min,水浴温度75℃。称取10mmol(0.633g)乙二胺和10mmol(0.913g)硝酸分别溶于5mL蒸馏水中,并滴加到底液中,搅拌加热1h后,减压蒸馏去除部分溶剂,过滤,得到0.65g金黄色固体,得率为23.5%。
元素分析(C5H12N10O4,%):计算值,C21.74,H 4.35,N 50.72; 实测值,C 21.84,H 4.31,N 50.81。IR(KBr,cm-1):3014(N─H),1640(N〖FY=,0.1mm〗N),1522(C〖FY=,0.1mm〗N); 1398,1313(C〖FY=,0.1mm〗N),1032,979(N─N); 822(NO-3); 1157(N+─H); 867(N─O),735(N─H)。Powder XRD(特征峰):10.5°、12.65°、18.45°、22.2°、23.9°、24.5°、25.65°、27.35°。
1.3 单晶培养将收集的母液保存于洁净的100mL烧杯内,封膜,在密封面上扎孔,静置于避光处,在室温下缓慢挥发得到不同粒度的单晶颗粒,挑选尺寸合适的单晶进行结构测试和分析。
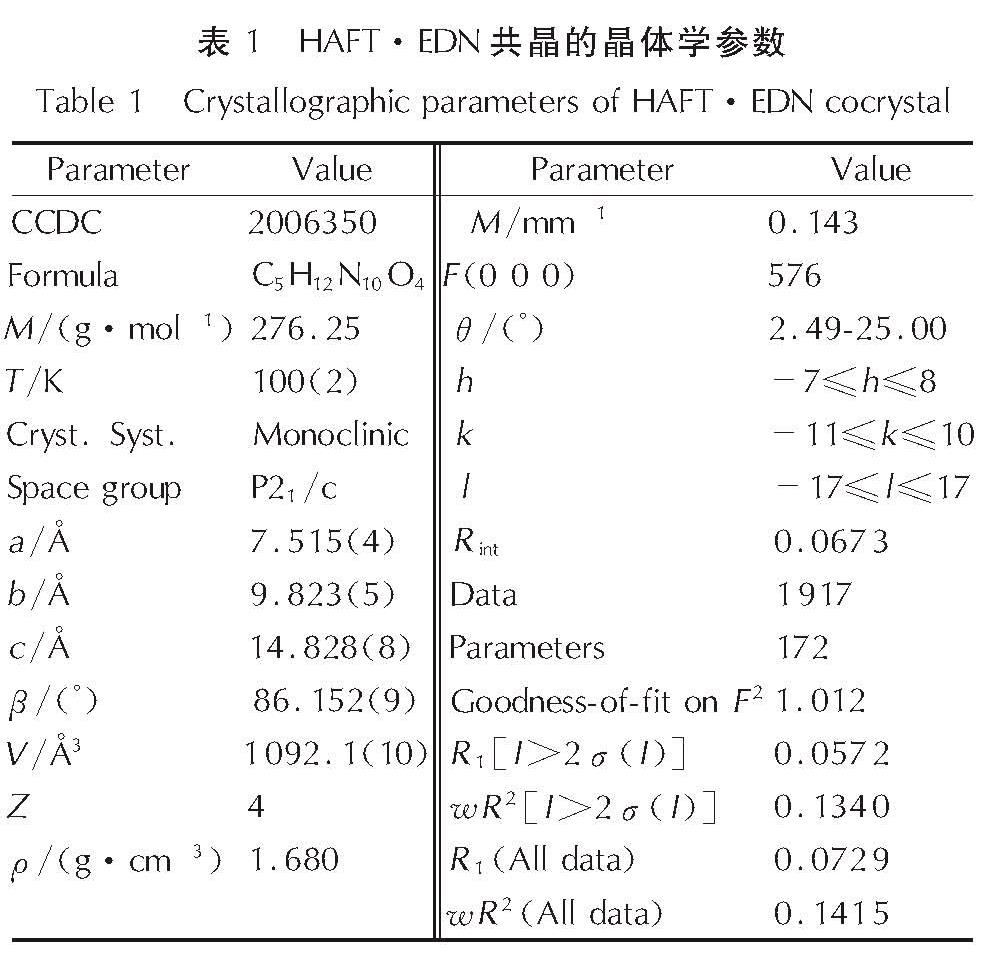
1.4 HAFT·EDN共晶的晶体结构测定选取尺寸0.33mm×0.25mm×0.21mm单晶,用Mo-Kα射线,石墨单色器,以ω扫描方式在100(2)K温度下进行扫描,共收集衍射点5294个,其中独立衍射点1917个(Rint=0.0673),晶体结构的解析使用SHELXS-97程序,并通过F2的全矩阵最小二乘法进行结构精修。全部非氢原子的坐标及各向异性热参数采用w=1/[σ2(Fo2)+(0.0298P)2+0.0000P],其中P=(F2o+2F2c)/3,经全矩阵最小二乘法修正及收敛。晶体学参数列于表1。
1.5 性能测试依据GJB772A-1997《炸药测试方法》中撞击感度测试方法测定HAFT·EDN共晶、HAFT、EDN的特性落高(H50),测试条件为:药量35mg,落锤质量2.5kg。
依据GJB772A-1997《炸药测试方法》中摩擦感度测试方法测定HAFT·EDN共晶、HAFT、EDN的爆炸概率,测试条件为:药量20mg,摆角90°,表压2.45MPa。
采用差示扫描量热仪(DSC)对共晶、HAFT、EDN进行热分析测试。DSC测试条件:样品质量为0.5mg,升温速率为5、10、15、20℃/min,温度范围0~500℃。
2 结果与讨论
3 结 论
(1)通过一步法将中性HAFT和离子型EDN合成得到了二元共晶HAFT·EDN,该共晶属于单斜晶系,空间群为P21/c,晶体密度为1.680g/cm3。晶体的堆积结构比较规则,HAFT分子内部的杂环基本平行,且晶体为面-面π-π堆积结构。
(2)二元共晶HAFT·EDN在升温速率10℃/min时,外延起始温度为279.5℃,放热峰的峰值温度为288.8℃,热爆炸临界温度为283.6℃。共晶的热抵抗能力较HAFT有所提高,热稳定性较EDN有所提高。
(3)二元共晶HAFT·EDN的机械感度低,安全性很高。
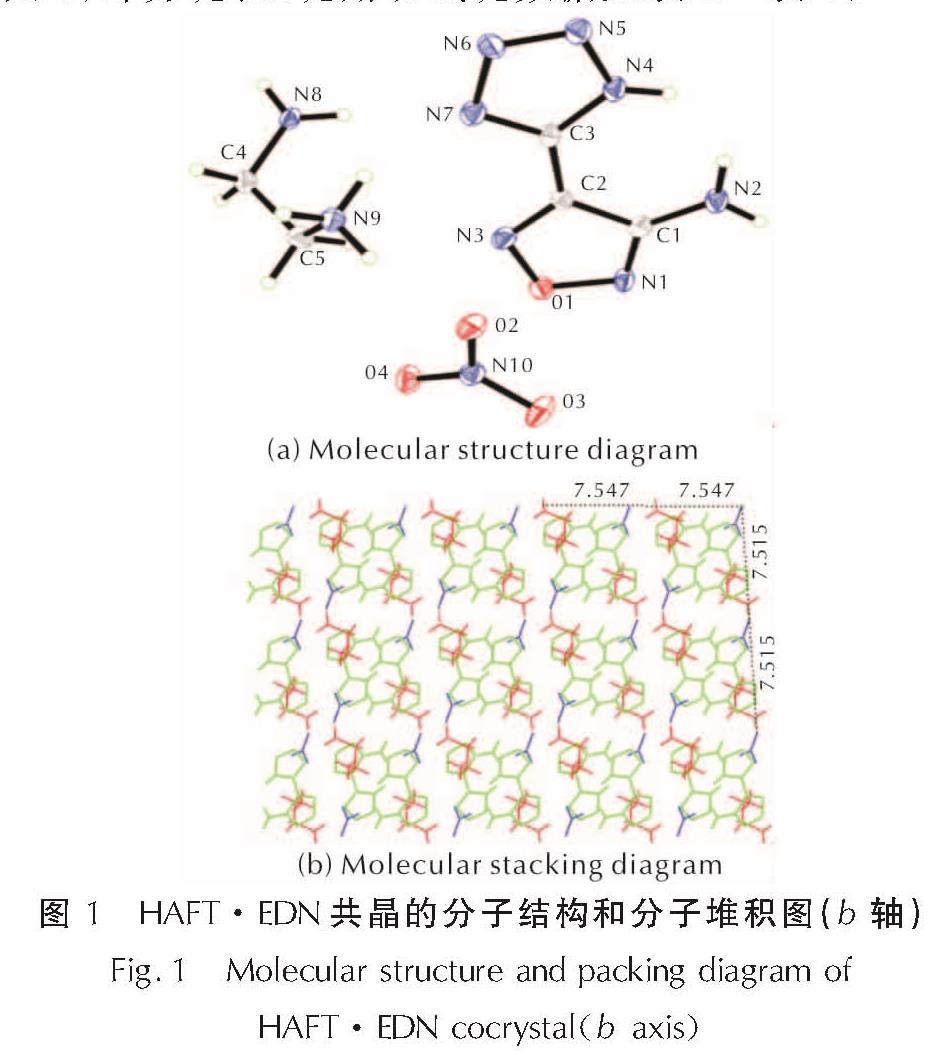
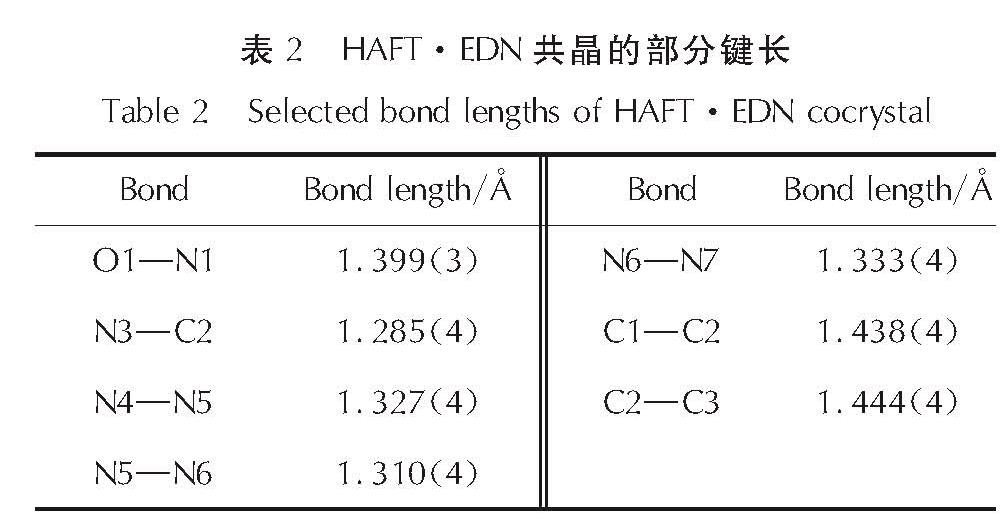
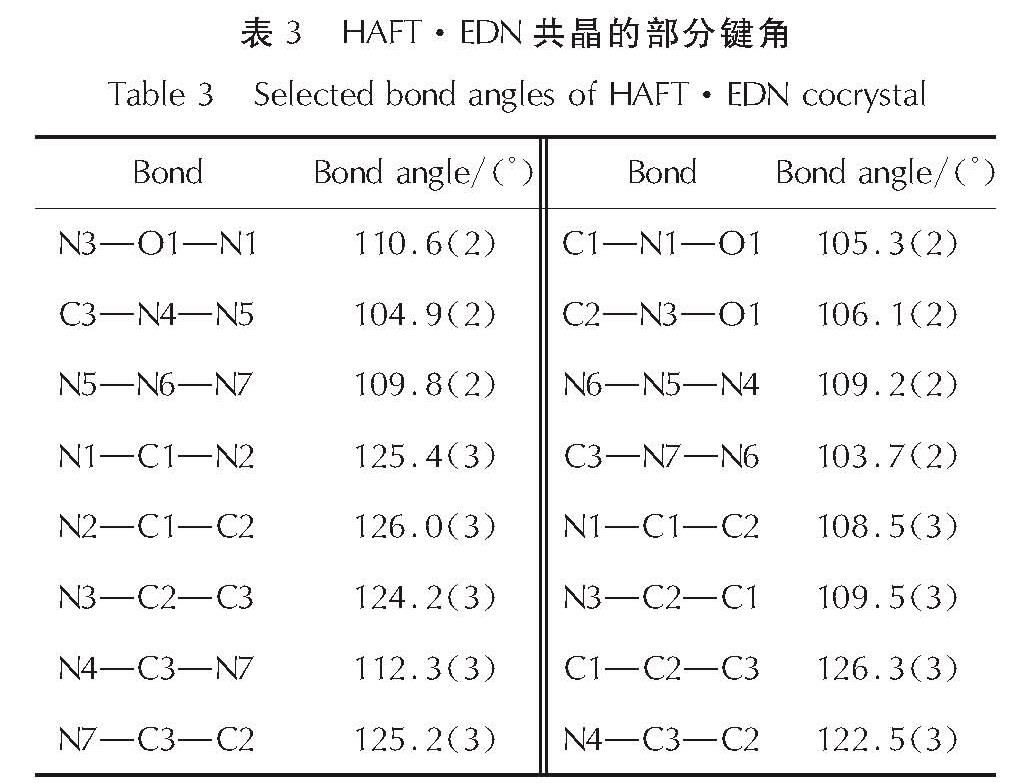
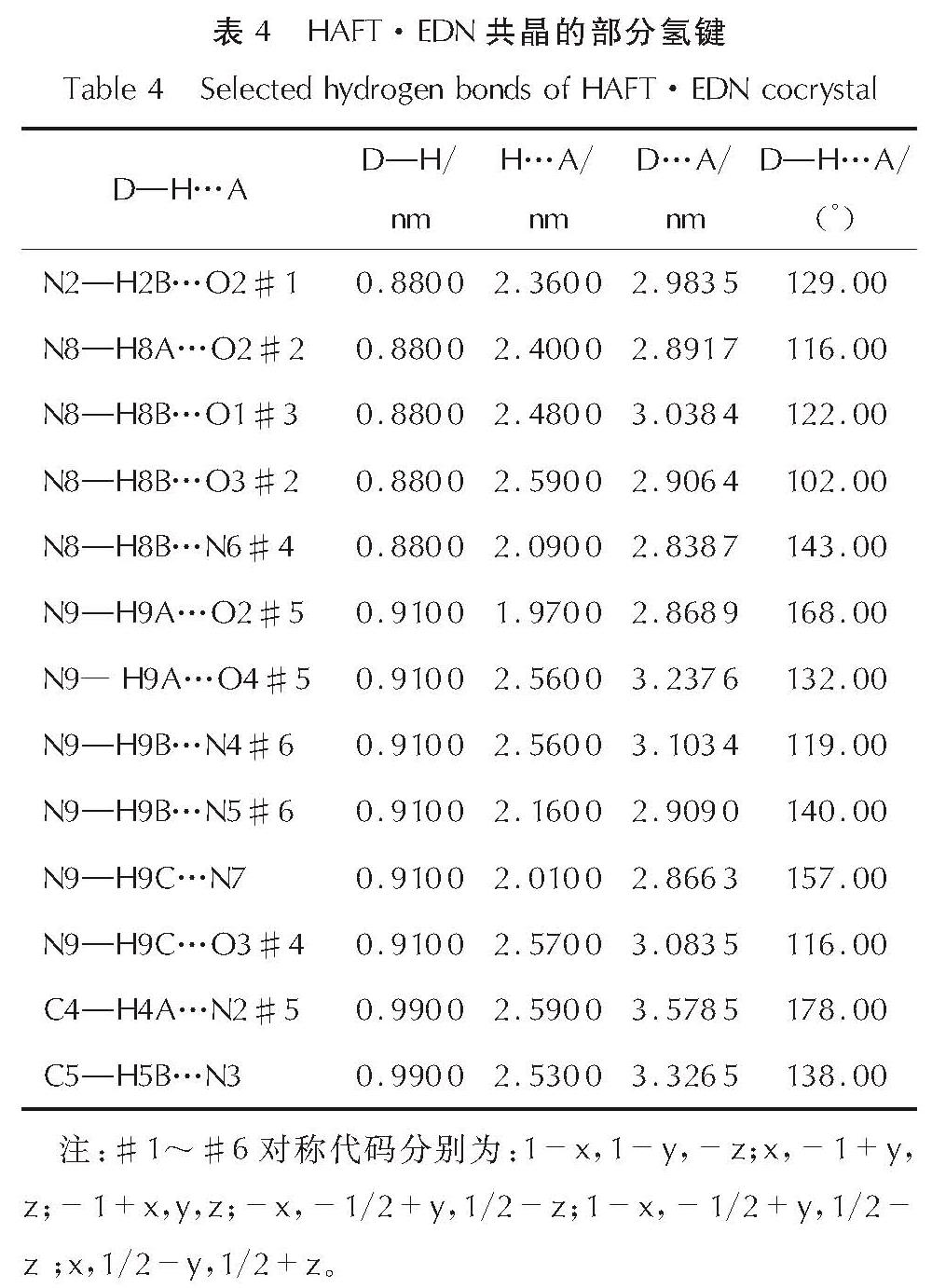
2.1 HAFT·EDN共晶的晶体结构分析HAFT·EDN共晶的分子结构和分子堆积图见图1; 部分键长、键角和氢键数据见表2~表4。
图1 HAFT·EDN共晶的分子结构和分子堆积图(b轴)
Fig.1 Molecular structure and packing diagram of HAFT·EDN cocrystal(b axis)由图1可以看出,其晶体结构单元由一个中性HAFT分子、一个硝酸乙二胺离子盐分子组成,且不含结晶水。其晶体密度为1.680g/cm3,而HAFT带一个结晶水,密度为1.612g/cm3。由表1可知,位于四唑环上的N─N键(1.310~1.333Å)的键长介于一般N─N单键(1.454Å)和N=N双键(1.245Å)之间。呋咱环上O1─N1的键长(1.399Å)比O1─N1的键长(1.371Å)要稍长,由此表明O1─N1键可能是热分解反应的触发键。四唑环的键长介于N5─N6(1.310Å)和N6─N7(1.333Å)之间,平均值为1.3236Å,比C=N键(1.270 )长0.0536Å、比N=N键(1.252Å)长0.0716Å,而比N─N键(1.470Å)短0.1464Å。呋咱环的键长介于N3─C2(1.285Å)和C1─C2(1.438Å)之间,平均值为1.358Å。
如图1(b)所示,共晶分子间距大约在7.5Å左右,且分子间氢键使其结构扩展成为一个以面-面π-π堆积[23]模式存在的3D结构,可有效缓冲外部机械刺激。HAFT分子内的四唑环和呋咱环是平行的,这也是HAFT作为中性母体的一个特征点之一,能够起到降低感度的作用。
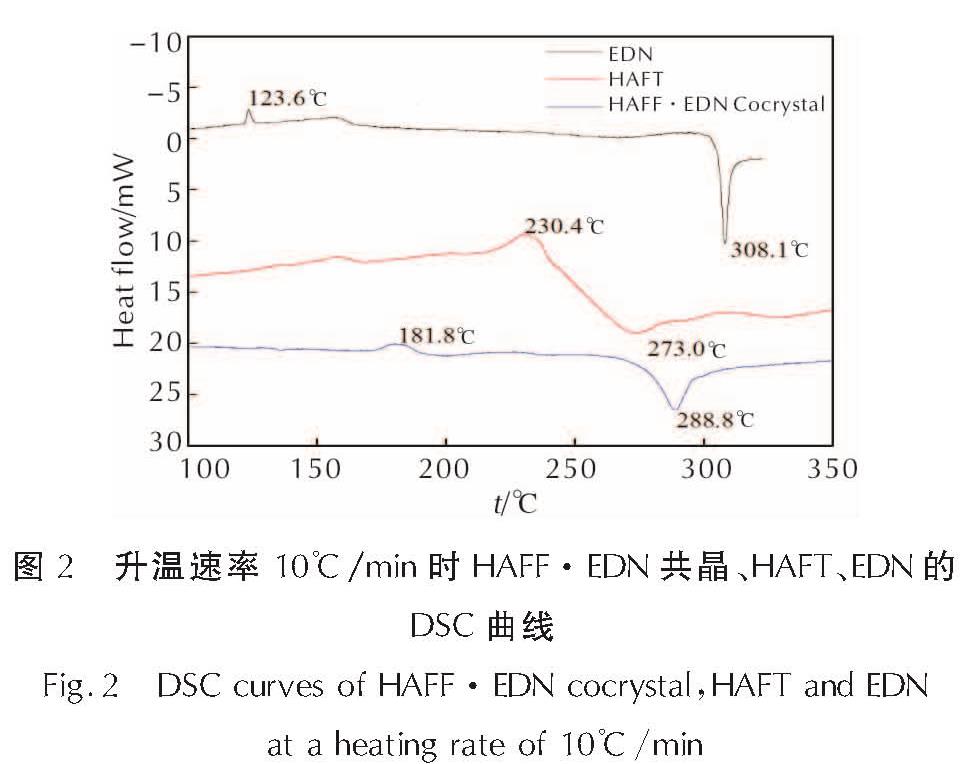
2.2 热分析2.2.1 HAFF·EDN共晶的热性能图2为HAFF·EDN共晶、HAFT、EDN在10℃/min的线性升温速率下的DSC曲线。
由图2可知,三者都存在一个吸热峰和一个放热峰,表明3种物质都经过了一个吸热熔化的相变过程和分解过程。HAFF·EDN共晶的融化吸热峰的
图2 升温速率10℃/min时HAFF·EDN共晶、HAFT、EDN的DSC曲线
Fig.2 DSC curves of HAFF·EDN cocrystal,HAFT and EDN at a heating rate of 10℃/min温度为181.8℃,放热峰的外延起始温度为279.5℃,峰值温度为288.8℃。HAFT的融化吸热峰的温度为230.4℃,峰值温度为273.0℃。EDN的融化吸热峰的温度为123.6℃,峰值温度为308.1℃。
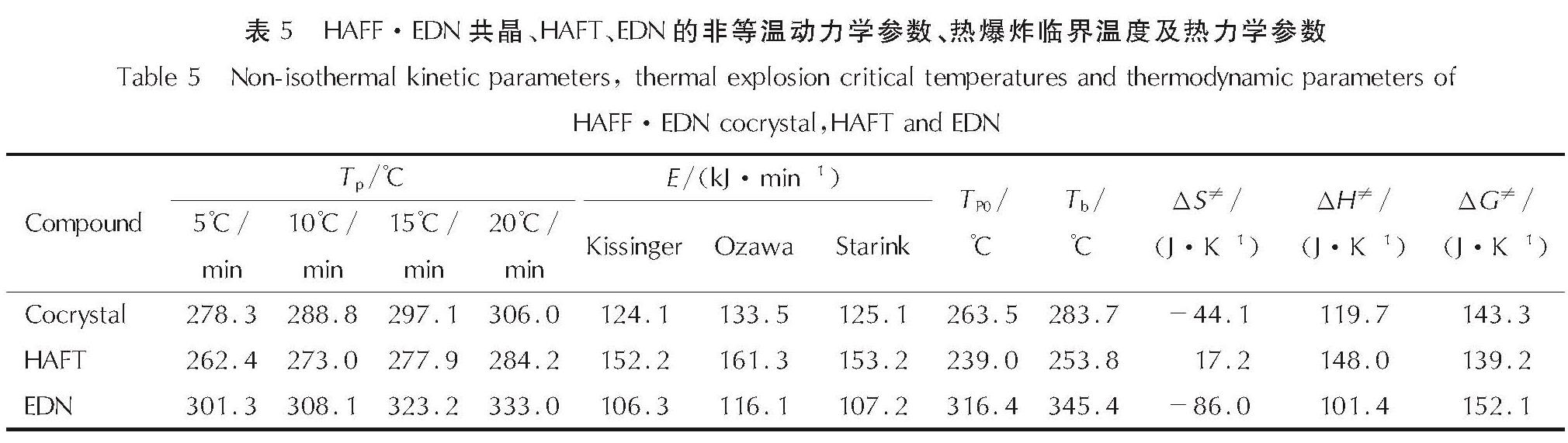
2.2.2 非等温动力学参数及热力学参数根据不同升温速率下分解时的第一放热峰温度,运用Kissinger法[24]、Ozawa法[25]和Starink法[26]研究了HAFF·EDN共晶、HAFT和EDN的非等温动力学参数,由此得到其表观活化能、指数因子等参数,并进行对比研究。
表5 HAFF·EDN共晶、HAFT、EDN的非等温动力学参数、热爆炸临界温度及热力学参数
Table 5 Non-isothermal kinetic parameters, thermal explosion critical temperatures and thermodynamic parameters of HAFF·EDN cocrystal,HAFT and EDN由式(1)可计算得到升温速率趋近于0条件下的峰值温度(TP0); 用式(2)可计算得到热爆炸临界温度(Tb); 由式(3)、式(4)、式(5)可以分别计算得到分解反应过程的活化熵(ΔS≠)、活化焓(ΔH≠)以及吉布斯自由能(ΔG≠)的数值,计算所得参数数据见表5。
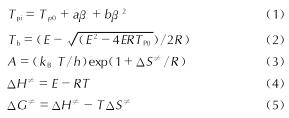
式中:a、b、c为系数; E=E^-; A=AK; kB为玻尔兹曼常数,1.381×10-23J/K; h为普朗克常数,6.626×10-34J·s。
将HAFT·EDN共晶与两个单体HAFT、EDN的热力学参数进行对比。看出HAFT·EDN共晶的热分解峰值温度比HAFT高24.5℃,而计算出来的表观活化能要低些,表明HAFT·EDN共晶的热稳定性较HAFT有所降低。HAFT·EDN共晶的热爆炸临界温度较HAFT升高了29.9℃,表明HAFT·EDN共晶的热抵抗能力较HAFT有所提高。与EDN相比,HAFT·EDN共晶的热分解峰值温度比EDN的低52.9℃,但计算出来的表观活化能要高些,表明共晶的热稳定性较EDN有所提高。HAFT·EDN共晶的热爆炸临界温度较EDN降低了61.7℃,表明HAFT·EDN共晶的热抵抗能力较EDN有所降低。同时三者的吉布斯自由能均大于0,证明热分解过程三者为非自发过程,需从外界吸收能量[27]。
2.3 感度测试测试得到HAFT·EDN共晶、HAFT、EDN的特性落高H50值均大于100cm,摩擦感度为0,表明HAFT·EDN共晶、HAFT、EDN对撞击和摩擦均钝感,都具有很高的安全性。
- [1] LARA-OCHOA F, ESPINOSA-PETREZ G.Cocrystalsdefinitions[J].Supra-molecular Chem,2007,19(8):553.
- [2]SHAN N, ZAWOROTKO M J. The role of cocrystals in phar-maceuticalscience[J].Drag Discovery Today,2008,13(9):440.
- [3]豆荣荣,ZERRAZA-SOFIANE,张教强,等. 含能共晶技术研究进展[J]. 材料导报,2017,31(9):90-96.
- [4]张坤,王晓峰,陶俊,等.共晶炸药的设计及制备研究进展[J].飞航导弹,2019(7):88-93.
- [5]马鹏,王金鹏,朱顺官.乙二胺-三乙烯二胺/甲胺-三乙烯二胺高氯酸盐共晶含能材料的制备和理论计算研究[J].化学研究与应用,2018,30(9):1451-1456.
- [6]舒远杰,武宗凯,刘宁,等.晶形控制及形成共晶:含能材料改性研究的重要途径[J].火炸药学报,2015,38(5):1-9.
- [7]刘可,张皋,陈智群,等.共晶含能材料研究进展[J].化学分析计量,2014,23(5):139-142.
- [8]黄晓川,郭涛,刘敏,等.联唑类含能化合物及其含能离子盐研究进展[J].含能材料,2015,23(3):291-301.
- [9]张兴高,朱慧,张炜,等. 呋咱类含能化合物及其在推进剂中的应用[J]. 化学推进剂与高分子材料,2006(3):1-5.
- [10]GAO W J, LIU X Y, SU Z Y,et al. High-energy-density materials with remarkable thermostabilityand insensitivity: syntheses,structures andphysicochemical properties of Pb(II)compounds with 3-(tetrazol-5-yl)triazole[J]. J Mater Chem. A, 2014, 2(30):11958-11965.
- [11]易潜洪, 黄明, 梁德辉,等.一种合成3-(4-氨基呋咱-3-基)-4-(4-硝基呋咱-3-基)呋咱的方法:CN,105566244A[P].2016.
- [12]于倩倩,刘耀鹏,李辉.3-硝基-4-(四唑-5-基)呋咱含能离子盐的合成及性能研究[J].应用化工,2015,44(9):1764-1766.
- [13]章雷. 呋咱四唑类化合物及单氧化呋咱的合成和性能预测[D].南京:南京理工大学,2016.
- [14]梁洁. 四唑基呋咱类HE-MOFs的分子设计、表征及性能研究[D].太原:中北大学,2017.
- [15]丁自美. 高氮四唑衍生物(HAFT)含能配合物的合成、表征、性能及理论研究[D].西安:西北大学,2019.
- [16]WU B D, BI Y G, LI F G, et al. A novel stable high-nitrogen energetic compound: copper(II)1,2-diaminopropaneazide[J]. Z Anorg Allg Chem,2014,640(1):224-228.
- [17]CHEN F, ZHENG F K, LIU G N, et al. Crystal structure and magnetic property of a 3D heterometalliccoordination polymer constructed by 3-cyanobenzoate and 3-(5H-tetrazol)benzoate ligands[J]. Inorg Chem Communications,2010, 13(2):278-281.
- [18]WANG S H,ZHENG F K, WU M F, et al. Assembly of Co(ii)/Cu(ii)-azidopolynuclearpolymers: structuraldiversity andmagnetic behavior[J]. Crystengcomm, 2014, 16(10):2009-2015.
- [19]WANG W, CHEN S,GAO S. Syntheses and characterization of lead(II)N,N-bis[1(2)H-tetrazol-5-yl]amine compounds and effects onthermal decompositionof ammonium perchlorate[J].Chem Ber, 2009(23):3475-3480.
- [20]XIA X, CHEN S, WEI Q, et al. Syntheses and characterization of energetic compounds constructed from alkaline earth metal cations(Sr and Ba)and 1,2-bis(tetrazol-5-yl)ethane[J]. J Solid State Chem, 2011, 184(7):1777-1783.
- [21]BADGUJAR D M, TALAWAR M B,ASTHANA S N, et al. Advances in science and technology of modern energetic materials: an overview[J]. J Hazard Mater, 2008, 151(2/3):289-305.
- [22]TALAWAR M B, SIVABALAR R,MUKUNDAN T, et al. Environmentally compatible next generation green energeticmaterials(GEMs)[J].J Hazard Mater, 2009, 161(2/3):589-607.
- [23]张朝阳. 含能材料能量-安全性间矛盾及低感高能材料发展策略[J]. 含能材料,2018,26(1): 2-10,123.
- [24]KISSINGER H E. Reaction kinetics in fifferential thermal analysis[J]. Analytical and Bioanalytical Chemistry, 1957, 29(11): 1702-1706.
- [25]OZAWA T. A new method of analyzing thermogravimetricdata[J].Bulletin of the Chemical Society of Japan,1965, 11(38): 1881-1886.
- [26]BOSWELL P G. On the calculation of activation energies using a modified kissingermethod[J]. Journal of Thermal Analysis and Calorimetry,1980, 38(11): 353-358.
- [27]陈腾,李强,郭双峰,等. GAP-HDI/CL-20纳米复合含能材料的制备、表征及其热分解特性[J]. 火炸药学报,2018,41(3):243-249.