作者简介:张坤(1992-),男,博士研究生,从事混合炸药技术研究。E-mail:zhangkun204@163.com 通信作者:王晓峰(1967-),男,博士,博导,研究员,从事混合炸药技术研究。E-mail:wangxf_204@163.com
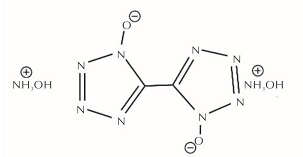
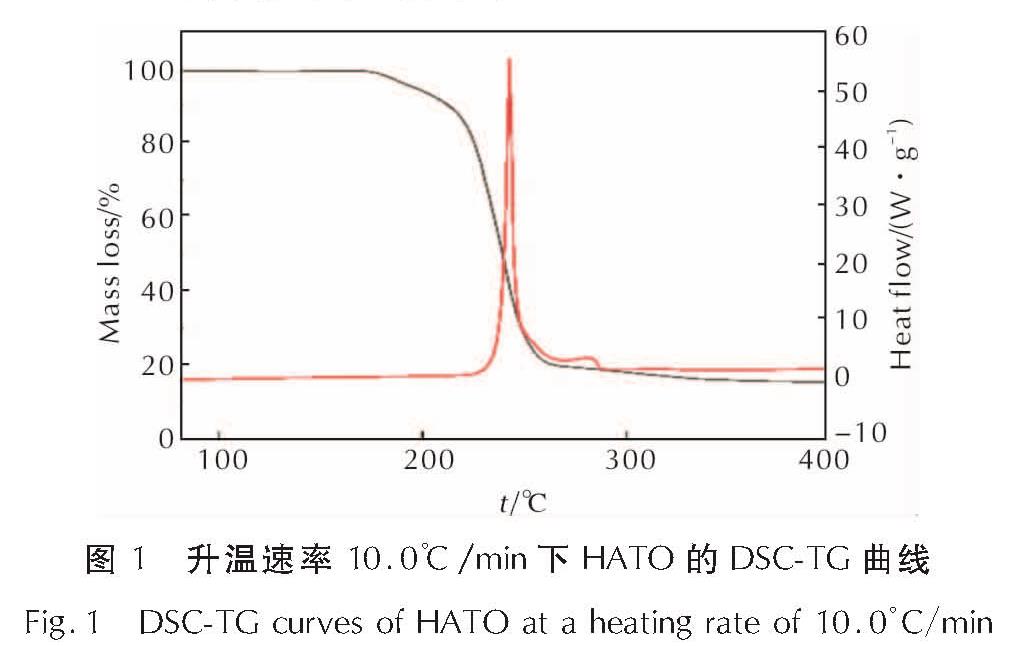
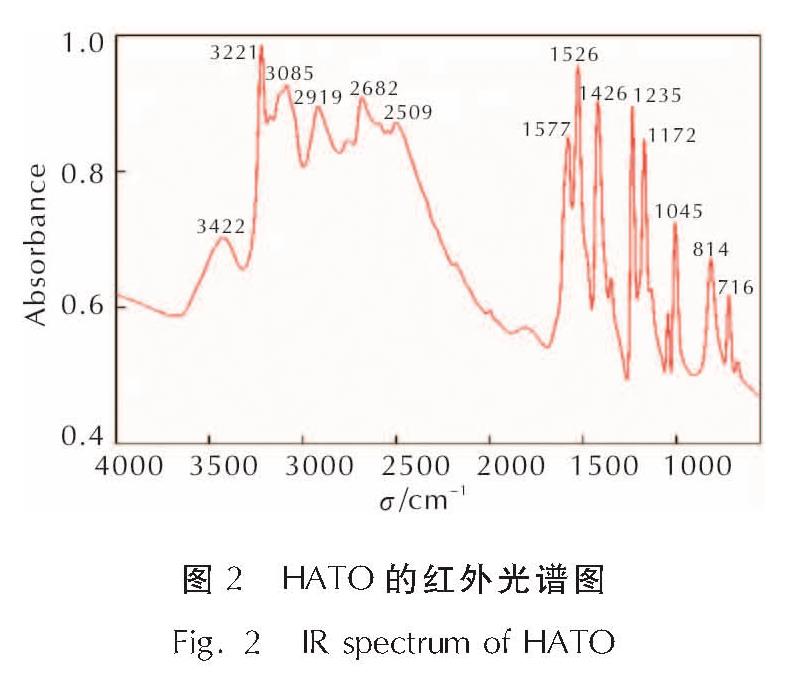
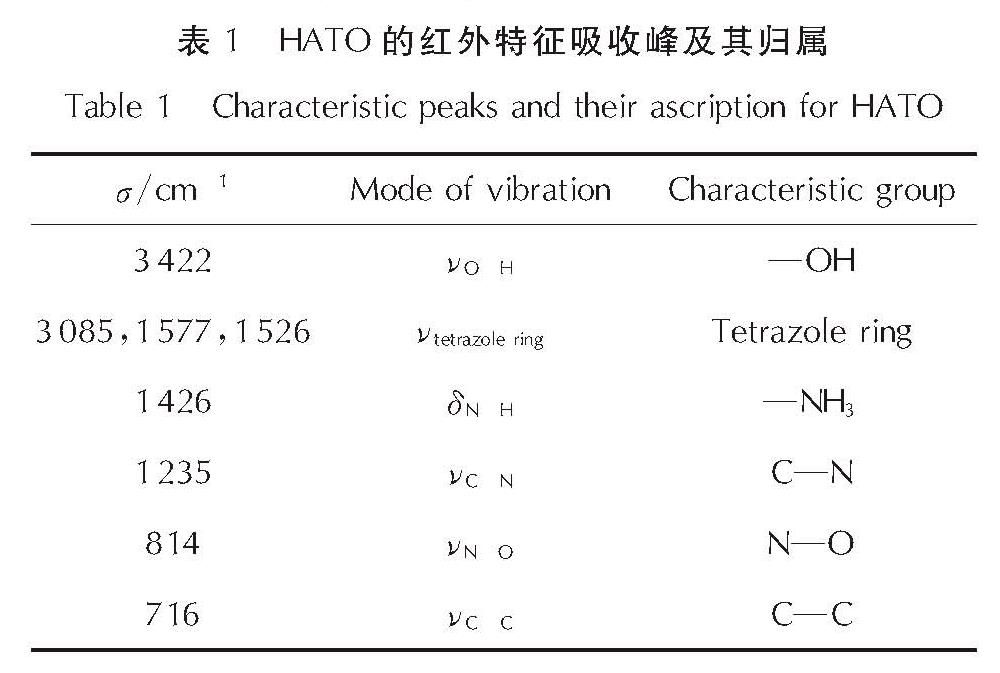
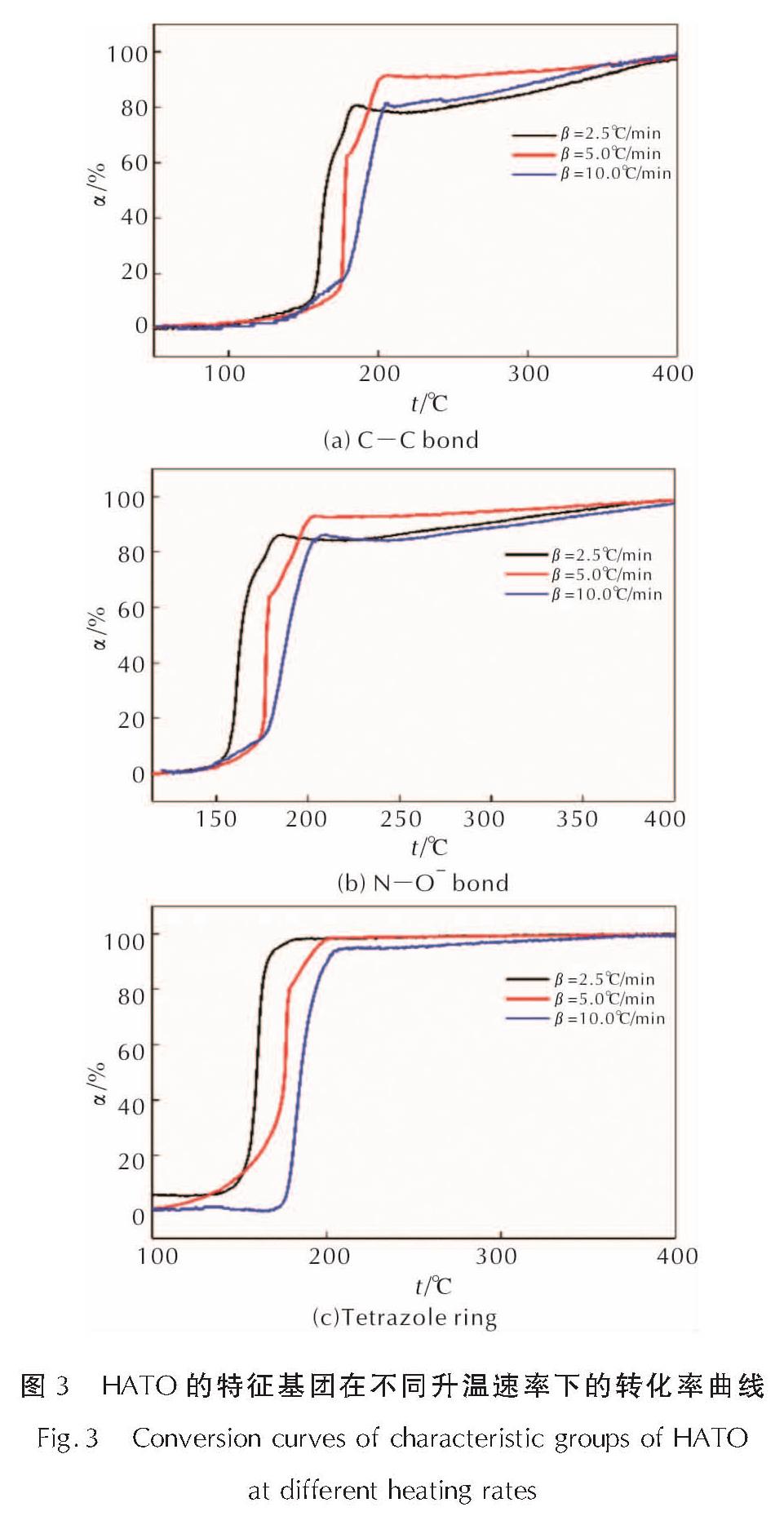
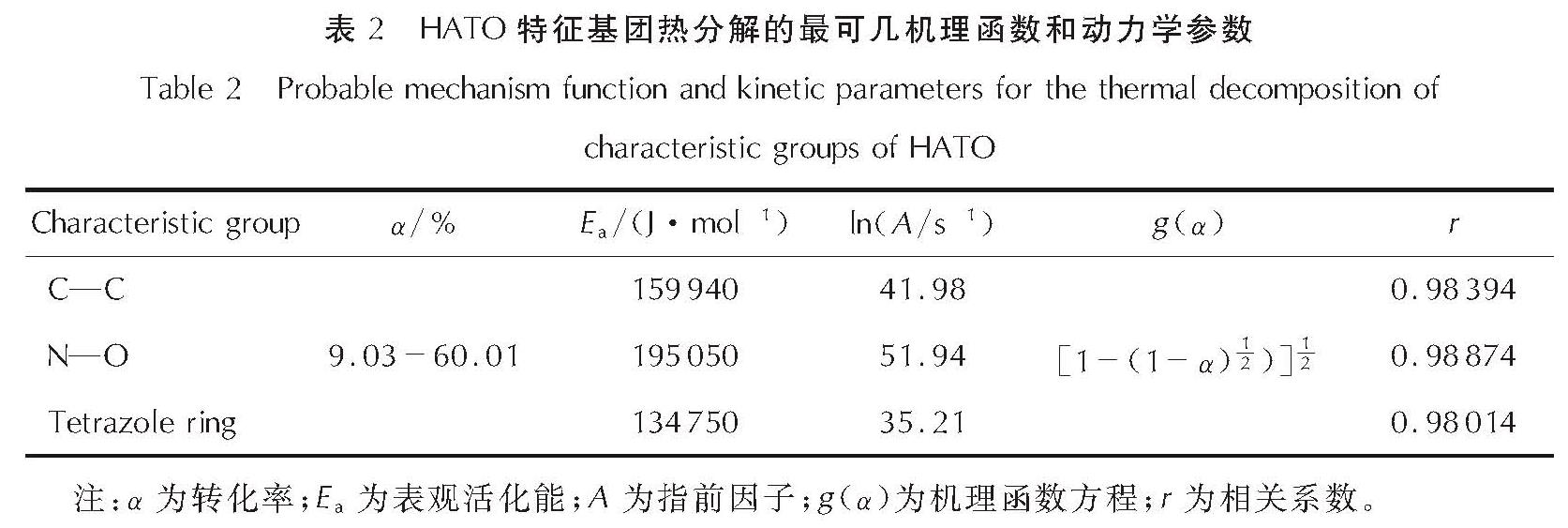
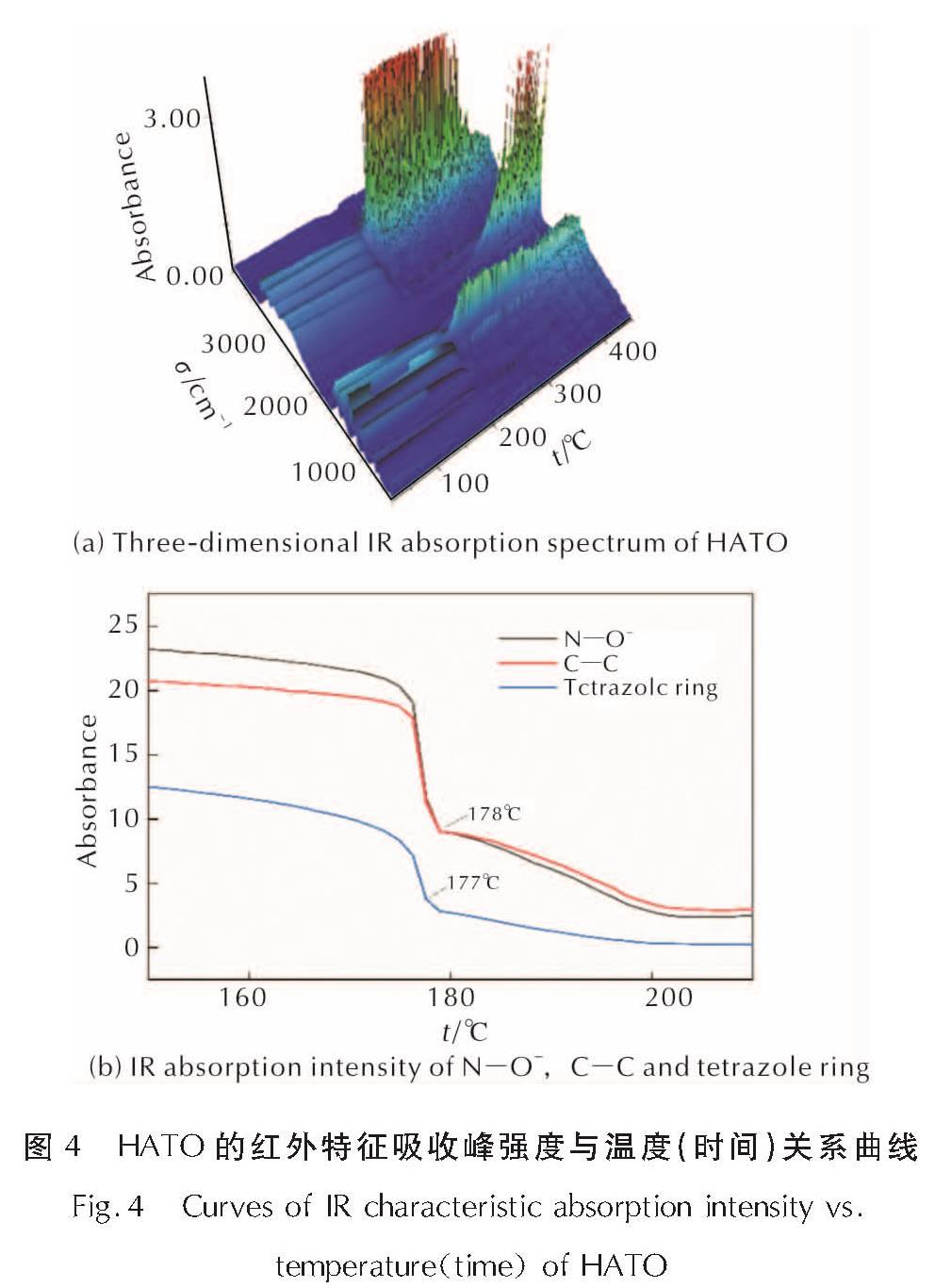
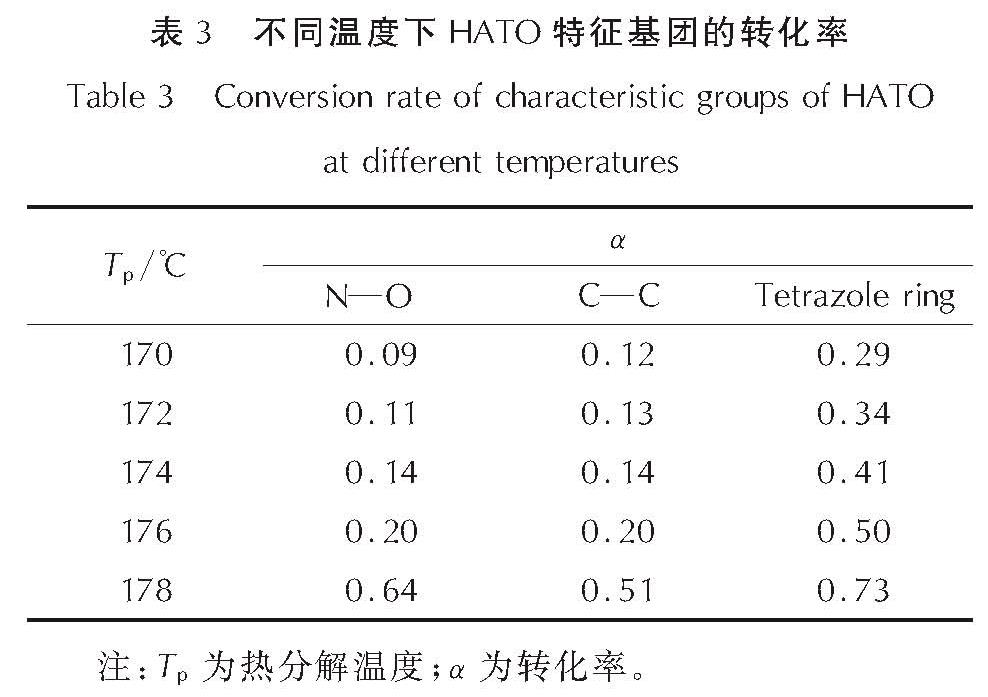
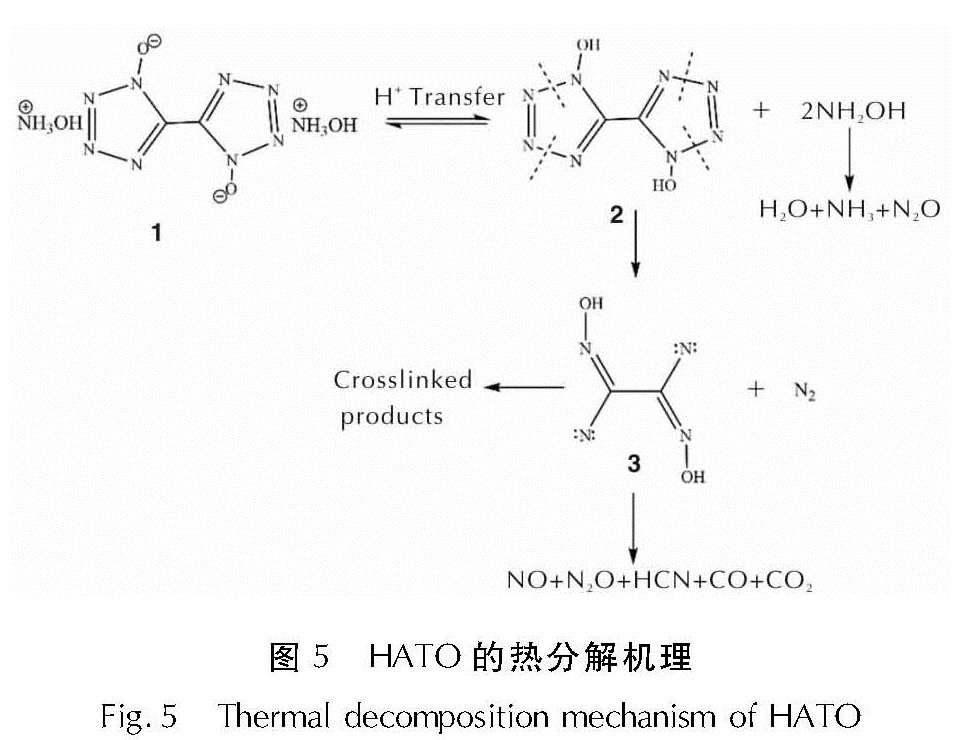
为详细了解5, 5'-联四唑-1, 1'-二氧二羟铵(HATO)及其特征基团的热分解特性,采用快速扫描傅里叶变换红外光谱法(RSFT-IR)、差示扫描量热法(DSC)分析了HATO的凝聚相热分解特性; 利用固体原位红外池/RSFT-IR技术在升温速率2.5、5.0、10.0℃/min下研究了HATO的特征基团随时间(温度)的变化; 采用Coats-Redfern法计算得到了HATO的C—C键、N—O-键、四唑环的热分解动力学参数; 基于HATO及其特征基团的热分解特性,推测了HATO的热分解机理。结果 表明,HATO及其特征基团的热分解受二维扩散机理控制,热分解过程分为两个阶段,第一阶段分解速率较快,第二阶段分解速率较慢且最终分解比较完全; HATO的C—C键、N—O-键、四唑环的活化能分别为159.94、195.05、134.75kJ/mol; 热分解过程为四唑环先断裂,然后伴随C—C键、N—O-键的断裂分解完全; HATO的热分解机理为:首先发生分子间质子转移,其次四唑环上的N—N键断裂和羟胺化合物进行分解,最后断裂碎片发生交联反应或继续受热分解为气体小分子达到分解完全。
To understand the thermal decomposition characteristics of dihydroxylammonium-5,5'-bis(tetrazole)-1, 1'-diolate(HATO)and its characteristic groups in detail,the thermal decomposition characteristics of condensed phase of HATO were analyzed by the rapid-scan fourier transform infrared(RSFT-IR)spectroscopy and differential scanning calorimeter(DSC). The variation of the characteristic group of HATO with time(temperature)was studied by the solid reaction cell in-situ/RSFT-IR at heating rates of 2.5, 5.0 and 10.0℃/min, respectively. The thermal decomposition kinetic parameters of C—C bond, N—O- bond and tetrazole ring of HATO were calculated by Coats-Redfern method. Based on the thermal decomposition characteristics of HATO and its characteristic groups, the thermal decomposition mechanism of HATO was deduced. Results show that the thermal decomposition of HATO and its characteristic groups is controlled by two-dimensional diffusion mechanism. The thermal decomposition process is divided into two stages. The decomposition rate of the first stage is faster, the decomposition rate of the second stage is slower and the final decomposition is complete. The activation energies of C—C bond,N—O- bond and tetrazole ring of HATO are 159.94, 195.05 and 134.75kJ/mol, respectively. The thermal decomposition process of HATO is that the tetrazole ring is broken first, and then with the breaking of C—C bond and N—O-bond, the decomposition is complete. The thermal decomposition mechanism of HATO is as follows: firstly, the intermolecular proton transfer reaction occurs, then, the N—N bond on the tetrazole ring is broken and the hydroxylamine compound is decomposed, and finally the broken fragments undergo a cross-linking reaction or continue to be decomposed into small gas molecules to achieve complete decomposition.