作者简介:胡松启(1976-),男,博士,教授,从事微推进技术研究。E-mail:pinecore@nwpu.edu.cn 通信作者:刘林林(1984-),男,博士,副研究员,从事固体推进剂技术研究。E-mail:lll@nwpu.edu.cn
(1.西北工业大学燃烧、热结构与内流场重点实验室,陕西 西安 710072; 2.西安航天动力技术研究所,陕西 西安 710025)
(1.Science and Technology on Combustion, Internal Flow and Thermo-Structure Laboratory, Northwestern Polytechnical University, Xi'an 710072, China; 2.Xi'an Aerospace Power Research Institute, Xi'an 710025,China)
physical chemistry; HAN; thermal decomposition; ferrocene derivative; PVA; kinetic parameters
DOI: 10.14077/j.issn.1007-7812.201907010
备注
作者简介:胡松启(1976-),男,博士,教授,从事微推进技术研究。E-mail:pinecore@nwpu.edu.cn 通信作者:刘林林(1984-),男,博士,副研究员,从事固体推进剂技术研究。E-mail:lll@nwpu.edu.cn
为了解决硝酸羟铵/聚乙烯醇(HAN/PVA)的点火和燃烧困难问题,采用热重-差示扫描量热-红外光谱-质谱联用技术研究了HAN/PVA的热分解机理,并通过多重扫描速率的非等温法探索了卡托辛、乙基二茂铁、辛基二茂铁和叔丁基二茂铁等对HAN/PVA热分解性能的影响。结果 表明,HAN/PVA的热分解主要发生在457.0~485.2K,失重73.7%,放热量为1312.1J/g,活化能Ea为88.8kJ/mol,在加热过程中固体样品基本转变成气态产物; 本研究所用二茂铁类衍生物对HAN/PVA的热分解均具有明显的催化效果,催化作用由强到弱依次为叔丁基二茂铁、辛基二茂铁、卡托辛及乙基二茂铁,分别使HAN/PVA的热分解温度降低了42.9、27.5、17.5和12.3K; 含辛基二茂铁、卡托辛和乙基二茂铁样品热分解反应的Ea分别为81.2、90.5和77.7kJ/mol; HAN/PVA/卡托辛样品热分解的Ea虽然最高,但指前因子A提高了2个数量级,反应速率常数大幅增加,催化效果最佳。
In order to increase the ignition and combustion performance of hydroxyl ammonium nitrate/polyvinyl alcohol(HAN/PVA), the thermal decomposition process of HAN/PVA was investigated by using simultaneous thermogravimetric analysis(TGA)-differential scanning calorimetry(DSC)-fourier transform infrared spectroscopy(FTIR)-mass spectrometry(MS), and the effect of catocene, ethylferrocene, octylferrocene and t-butylferrocene on the thermal decomposition of HAN/PVA was studied by multiple scanning methods. The results show that the decomposition process of HAN/PVA mainly occurs at 457.0-485.2K, with the mass loss, heat release and activation energy Ea of 73.7%, 1312.1J/g and 88.8kJ/mol respectively, and the solid samples mostly decomposed into gaseous products. The four ferrocene derivatives have obvious catalytic effects on the thermal decomposition of HAN/PVA, and the descending order of the catalysts effects is as follows: t-butylferrocene, octylferrocene, catocene and ethylferrocene, which make the decomposition temperature decrease by 42.9, 27.5, 17.5 and 12.3K, respectively. The Ea of HAN/PVA thermal decomposition with octylferrocene, catocene and ethylferrocene are 81.2, 90.5 and 77.7kJ/mol, respectively. The Ea of HAN/PVA/Catocene is the highest, however, the pre-exponential factor(A)increases 2 magnitudes,and the reaction rate constant greatly increased, leading to the best catalytic effect induced by catocene.
引言
微推进系统能够实现微小航天器的姿态调整、轨道控制和重力补偿等,固体化学微推进系统以固体推进剂为能源,具有结构简单、体积小和质量轻等优点,应用前景广阔[1-2]。但常规固体推进剂用于制备微推进剂药柱存在燃烧效率较低和生产安全性差等方面的问题,难以满足微推进系统的装药要求。硝酸羟铵(HAN)极易溶于水,采用HAN水溶液作氧化剂更易于实现微药柱的制备,且微药柱的燃烧受原材料的尺度效应影响小,有望实现高效燃烧,是一种较为理想的微固体推进剂用氧化剂。考虑到水溶性聚乙烯醇(PVA)常与HAN溶液配合作为凝胶推进剂和电控推进剂的黏合剂,故可采用PVA作为HAN基微固体推进剂的黏合剂[3-5]。
然而,硝酸羟铵/聚乙烯醇(HAN/PVA)推进剂在制备过程中PVA与水发生溶胀,从而水将氧化剂与燃料隔绝开来,且黏合剂的反应活性低,皆可能导致推进剂点火和燃烧困难。在推进剂点火过程中,点火药产生的燃气使推进剂燃面升温而发生热分解,分解产物进行强烈的氧化还原放热反应,产生火焰并最终实现推进剂的点燃。火焰向推进剂内部传播的过程即燃烧过程[6]。因此推进剂的点火燃烧起始于热分解,若对推进剂的热分解过程进行有效催化将有助于其点火燃烧性能的提升。
深入认识HAN的热分解机理是开展HAN/PVA推进剂热分解催化研究的基础。目前一般认为固体HAN的分解为质子传递反应,起始分解温度约为453K; HAN溶液的热分解为自催化过程,其分解会受到水的初始浓度影响; HAN的分解产物包括N2O、NO等氧化性气体,NO2是一种主要的中间产物[7-10]。在HAN基推进剂催化方面,通常采用铱和铂等贵金属来催化点火,但需要将催化床预热至473K,低温下点火延迟期长甚至无法点火,且推进剂较多积存在催化床上,导致发动机的启动性能受到限制。Popa、Kappenstein和Courthéoux等[11-14]在HAN催化分解的基础研究中取得了重要进展,采用溶胶-凝胶法和二氧化碳超临界干燥法制备了基于Al2O3的载体和催化剂,并引入质量分数5%的Pt金属,能够实现HAN水溶液在313K以下催化分解。此外,HAN基推进剂和催化剂匹配性问题也十分重要,匹配性不佳的配方会产生催化床空腔、催化剂寿命下降和试车稳定性差等问题,影响发动机的使用。
胡建新等[15]在HAN/PVA电控推进剂的研制过程中,加入二茂铁及其衍生物来提高推进剂的燃速,但这类催化剂对推进剂热分解特性的影响规律却未见报道,考虑到推进剂的点火燃烧起始于热分解,因此本研究选择二茂铁类衍生物作催化剂,采用热分析仪对HAN/PVA的热分解机理和动力学参数进行分析讨论,研究结果可为解决HAN/PVA的点火和燃烧困难问题提供帮助。
1 实 验
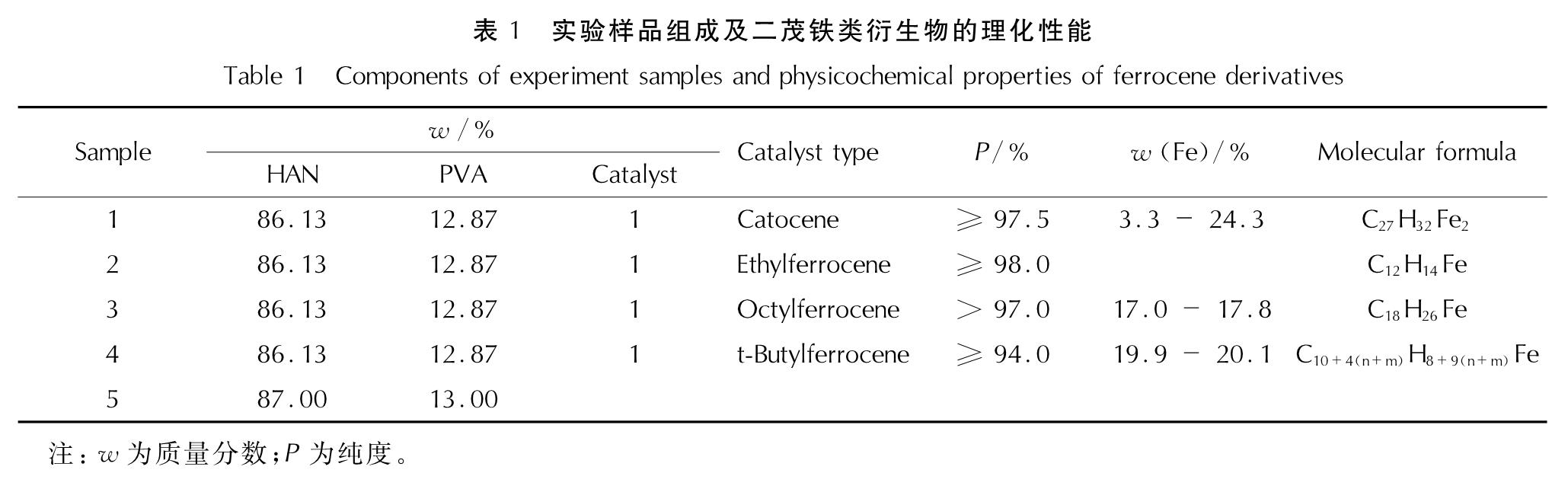
1.1 试剂与仪器HAN水溶液,质量分数80%,通过复分解法自制。PVA,化学纯,醇解度99.8%~100%,分子质量89000~98000,天津市科密欧化学试剂有限公司; 卡托辛(Catocene)、乙基二茂铁(Ethylferrocene)、辛基二茂铁(Octylferrocene)、叔丁基二茂铁(t-Butylferrocene),营口天元化工研究所股份有限公司。实验样品组成及二茂铁类衍生物主要理化性能见表1。
TGA/DSC1同步热分析仪,瑞士梅特勒-托利多公司; Tensor27红外光谱仪,德国布鲁克光谱仪器公司; OMNI star质谱仪,德国皮埃尔真空仪器公司。
1.2 HAN/PVA样品制备通过热力学计算可知,在燃烧室压强为5MPa、压强比pc/pe为50的条件下,当HAN水溶液和PVA质量比为87:13时,推进剂的理论比冲最高,选用该配比本实验制备了5个样品,制备过程为:首先将HAN水溶液和PVA混合,在338.2K下搅拌1~2h,直至药浆中无PVA微小颗粒,在室温条件下将质量分数为1%的二茂铁类衍生物加入样品1~样品4中并充分搅拌; 然后将药浆在338.2K下真空熟化1~2d后将温度降至308.2K成型,制得HAN/PVA样品。样品1~样品5的组成见表1。
1.3 热分析实验本研究首先采用TGA-DSC-FIRT-MS联用仪对22.8mg的HAN/PVA样品进行测试,测试氛围为氩气,温度范围为313.2~973.2K,升温速率为10K/min。然后利用TGA/DSC同步热分析仪,对样品1~样品5分别在升温速率7、10、15和25K/min条件下进行热分析测试,试样质量为(3 ± 0.1)mg,温度范围为308.2~623.2K。
2 结果与讨论
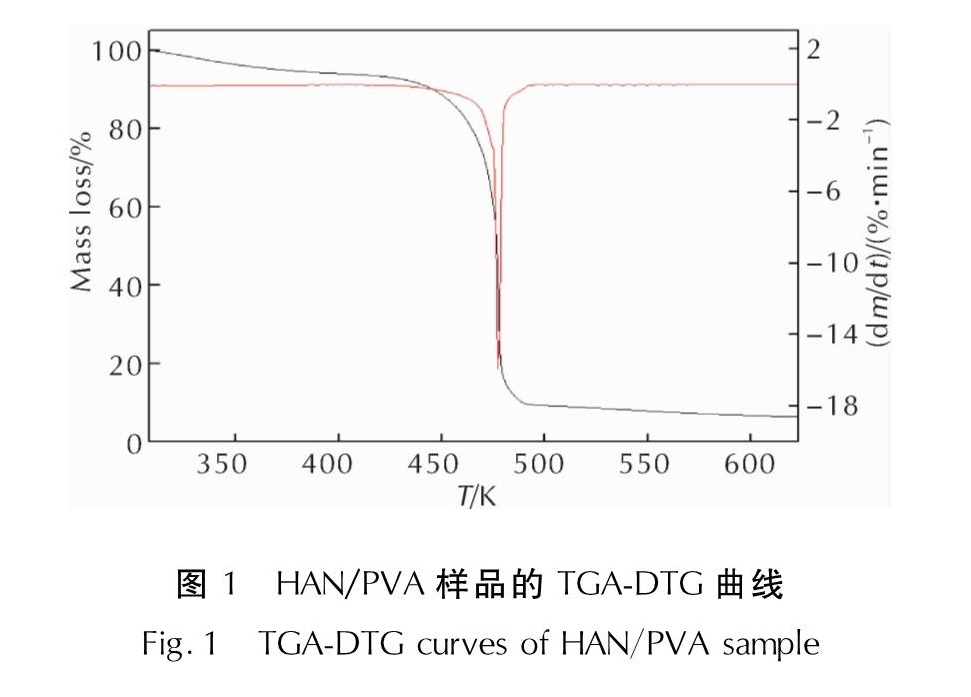
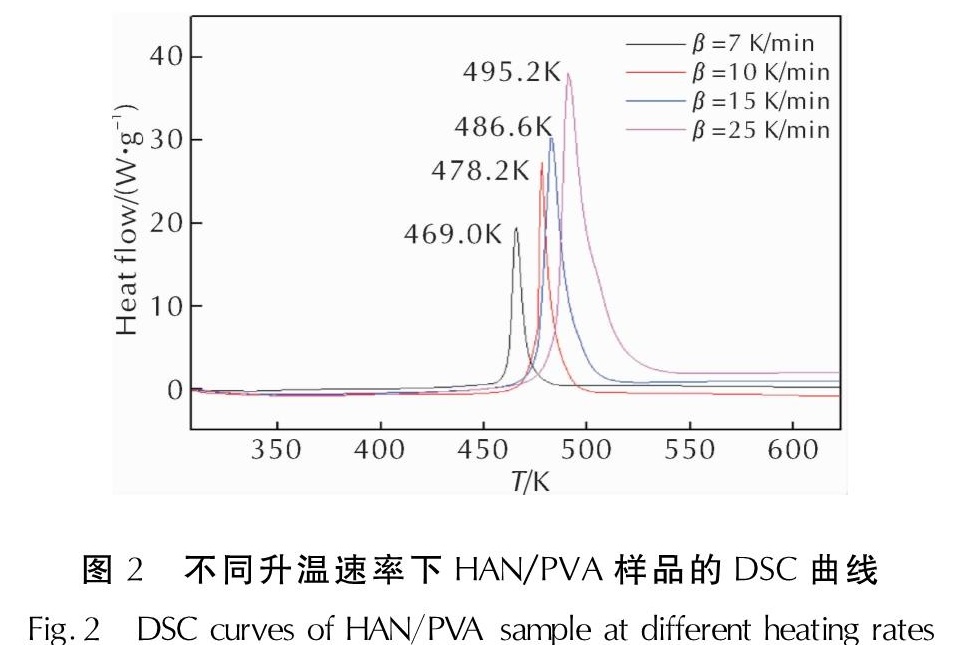
2.1 HAN/PVA热分解过程升温速率为10K/min时,HAN/PVA样品的TGA-DTG测试结果如图1所示。HAN/PVA样品在升温速率7、10、15和25K/min条件下测得的DSC曲线如图2所示。
由图1和图2中10K/min的DTG和DSC结果可知,HAN/PVA样品热分解仅有一个明显的放热分解峰,起始分解温度为457.0K,峰值温度为478.2K,终止分解温度为485.2K,由此推测HAN/PVA的热分解过程是一个固态分解过程,在此过程中样品的质量迅速降低,失重率约为73.7%,放热量约为1312.1J/g。由于PVA高分子的热分解通常发生在533~553K左右,因此该阶段的失重由HAN的热分解速度控制,快速失重和尖锐放热峰表明HAN在短时间内放出了大量的热。由图2可知,随着升温速率加快,HAN/PVA样品的起始分解温度和终止分解温度均升高,DSC曲线上的分解峰值温度升高,峰面积也变大,即放热量增大。
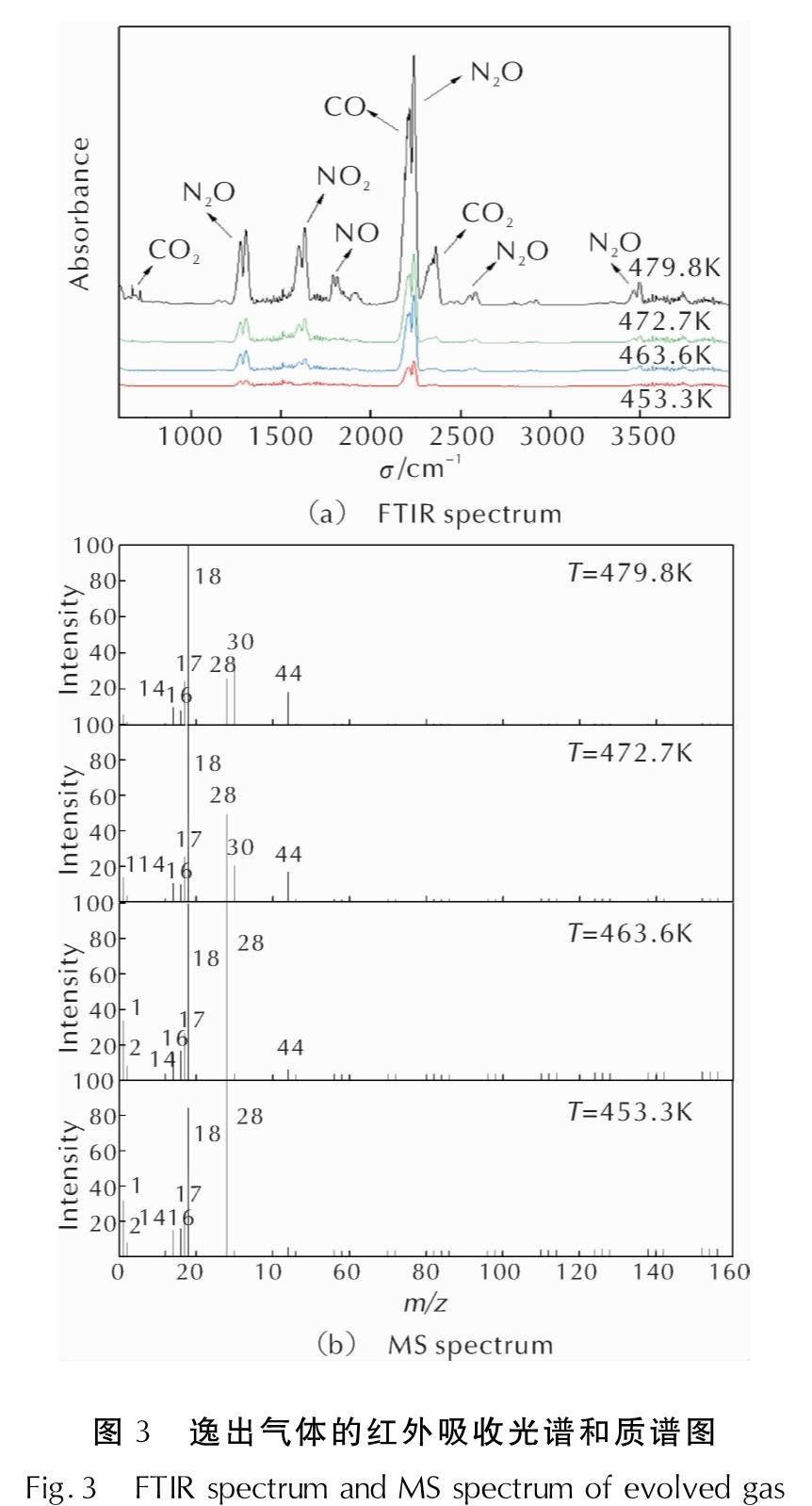
结合TGA-DSC-FIRT-MS联用仪测得的红外光谱和质谱结果,进一步研究HAN/PVA的热分解过程。不同温度下HAN/PVA热分解逸出气体产物的红外光谱图和质谱图如图3所示。通过与标准红外吸收光谱比对,在图3(a)中标出各吸收峰代表的分解产物。
从图3(a)可知,1200~1350、2200~2300、2550~2650和3400~3550cm-1处为N2O的红外光谱吸收峰,2000~2250cm-1处为CO的红外光谱吸收峰,1500~1700cm-1处为NO2的红外光谱吸收峰,500~750和2250~2400cm-1处为CO2的红外光谱吸收峰,1750~2000cm-1处为NO的红外光谱吸收峰[16-17]。图3(b)中,质荷比m/z=18的质谱峰为H2O,m/z=17主要为NH3和H2O的离子碎片OH+,m/z=1主要为H2O的离子碎片H+,m/z=2的质谱峰为H2,m/z=44的质谱峰主要包括N2O和CO2,m/z=16的质谱峰主要为NH3的离子碎片NH+2和CO2的离子碎片O+,m/z=30的质谱峰为NO,m/z=28的质谱峰主要包括CO和N2,m/z=14的质谱峰主要为N2的离子碎片N+。
由图3可知,450~464K范围内,HAN/PVA热分解首先生成了H2O、H2、CO、NH3、N2O及NO2,N2O分解产生N2并将部分H2氧化成H2O; 温度逐渐升高至473K左右,H2基本被氧化成H2O,气体产物中H2O和N2O的含量增大,NO2发生分解生成了NO,并将NH3和部分CO氧化为NO和CO2; 最终当温度升高至480K左右时,NO的含量超过了CO和N2。HAN/PVA样品热分解的气相产物中,H2、CO、N2O和NO的产生可归因于配方的氧燃比偏小。
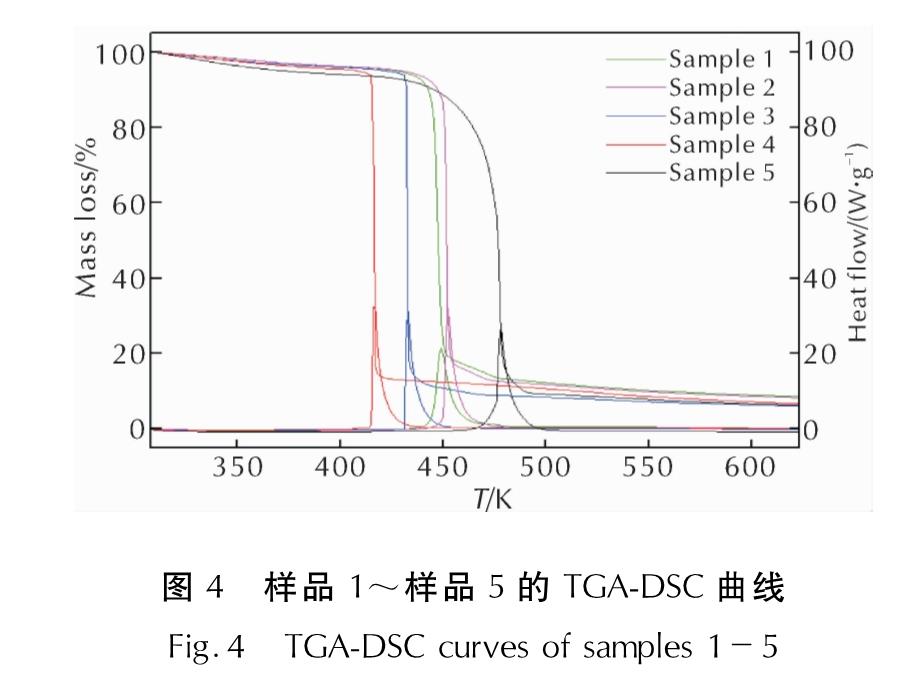
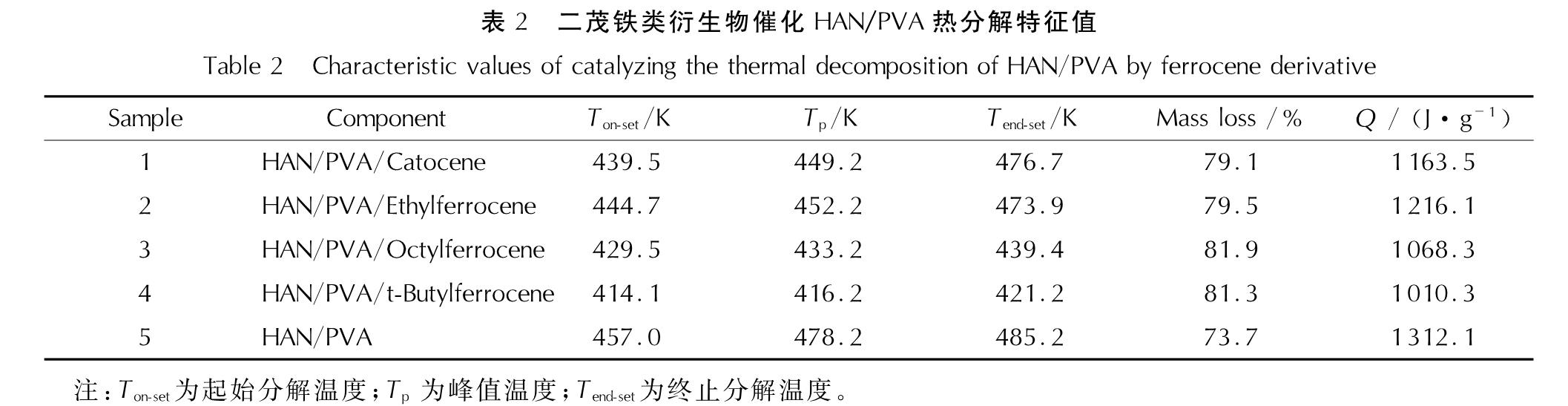
2.2 二茂铁类衍生物对HAN/PVA热分解过程影响HAN/PVA存在点火困难问题,本研究在配方中添加了质量分数1%的卡托辛、乙基二茂铁、辛基二茂铁或叔丁基二茂铁作催化剂,以期改进HAN/PVA的点火燃烧性能。升温速率为10K/min时,样品1~样品5的TGA-DSC曲线如图4所示,热分解相关的特征值见表2。
表2 二茂铁类衍生物催化HAN/PVA热分解特征值
Table 2 Characteristic values of catalyzing the thermal decomposition of HAN/PVA by ferrocene derivative图4曲线表明,加入质量分数1%的二茂铁类衍生物后,样品1~样品4的热分解过程与HAN/PVA相同,热分解的DSC曲线均仅有一个明显的放热分解峰。表2中数据显示,样品1~样品5的起始分解温度和分解峰值温度从低到高依次为:样品4<样品3 <样品1<样品2<样品5,终止分解温度为:样品4<样品3<样品2<样品1<样品5,热分解持续时间为:样品4<样品3<样品5<样品2<样品1。上述结论表明,叔丁基二茂铁和辛基二茂铁加快了HAN/PVA热分解速率,反应进行地比较完全; 卡托辛和乙基二茂铁虽然使HAN/PVA的热分解温度提前,但是样品热分解产生的固相产物覆盖在催化剂表面,可能使其反应活性降低,导致了主反应后期的热分解速率下降,反应时间变长,反应不够完全,残余量略大。当升温速率为7、15或25K/min时,5种样品的热分析曲线表现出相同的规律,即4种二茂铁类衍生物对HAN/PVA热分解的催化顺序不受升温速率的影响。
此外,从表2可以看出,样品1~样品4的失重率比样品5高,且样品的热分解温度大幅提前,因此二茂铁类衍生物能够有效促进HAN/PVA的热分解反应。研究表明,二茂铁类衍生物对固体推进剂的催化作用主要发生在气相中,在凝聚相界面上也有催化作用[18]。因此,在样品热分析的升温过程中,二茂铁类衍生物首先失去茂环上的取代基,生成不稳定的二茂铁正离子,HAN内部发生质子传递过程并开始分解; 然后二茂铁正离子分解成裸露的金属离子或被氧化成粒度更小的Fe2O3,这些产物靠近样品表面并催化HAN/PVA的热分解过程,HAN/PVA的初始分解产物继续分解生成了CO、CO2、N2、NO、NO2、N2O和H2O,反应放出的部分热量加热样品从而进一步加快了热分解反应进程。表2中放热量数据显示,4种二茂铁类衍生物催化剂的加入均使得HAN/PVA热分解的放热量降低,减少量在7.3%~23.0%之间,这可能是因为催化分解反应放出的热量积聚在气相中,导致了系统可测得的放热量偏低。
图4和表2中5种配方的TGA和DSC测试结果表明,二茂铁类衍生物使得HAN/PVA的热分解温度显著降低,因此具有明显的催化效果,其中叔丁基二茂铁的催化效果最好,之后依次为辛基二茂铁、卡托辛及乙基二茂铁,使HAN/PVA的分解温度分别降低了约42.9、27.5、17.5和12.3K。
2.3 HAN/PVA热分解动力学参数分析图4中样品4的热分解曲线表明,加入叔丁基二茂铁后样品的热分解反应瞬间完成,已经不受动力学控制,因此本研究对样品1~样品3和样品5的4种配方样品的主反应区进行动力学分析。根据式(1)将样品在升温速率为7、10、15和25K/min时测得的TGA曲线变换为转化率和温度关系曲线(α—T曲线)。
α=(W0-WT)/(W0-W)(1)
式中:α为转化率; W0为初始质量百分数; WT为温度T时的质量百分数; W为热分解结束时的剩余质量百分数。
以0.05为间距,在0.3<α<0.8范围内,求得4条α—T曲线上不同α值对应的温度Tα,并利用等转化率法可求出各转化率αi下的活化能值。本研究利用Flynn-Wall-Ozawa法、Kissinger-Akahira-Sunose法和Starink法3种无模型法计算4种样品的活化能Ea,通用式分别为式(2)、式(3)和式(4)[19-21]:
In(β)=Const-1.052((Ea)/(RT))(2)
In(β/(T2))=Const(Ea)/(RT)(3)In(β/(T1.92))=Const-1.0008((Ea)/(RT))(4)
式中:β为升温速率,K/s。
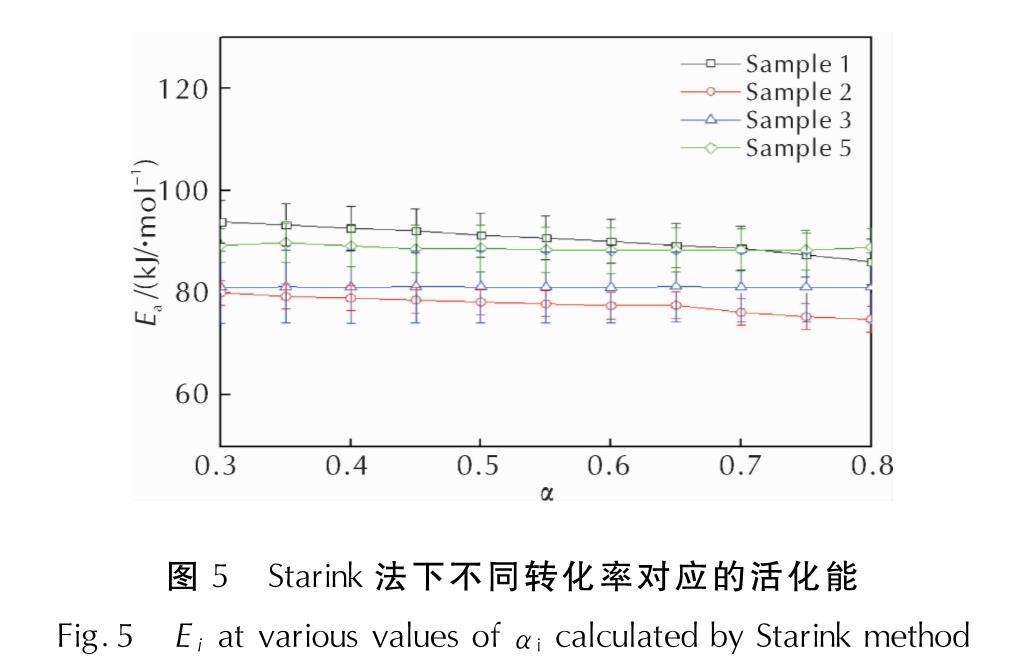
Flynn-Wall-Ozawa法拟合ln(βi)-1/Ti、Kissinger-Akahira-Sunose法拟合ln(βi/Ti2)-1/Ti、Starink法拟合ln(βi/Ti1.92)-1/Ti来确定各转化率αi下的活化能Ei。将每种方法在不同转化率αi下的Ei作图,由于3种方法的Ei变化趋势一致,故本研究仅展示Starink法求解得到的4种样品热分解过程αi所对应的Ei,如图5所示。
图5 Starink法下不同转化率对应的活化能
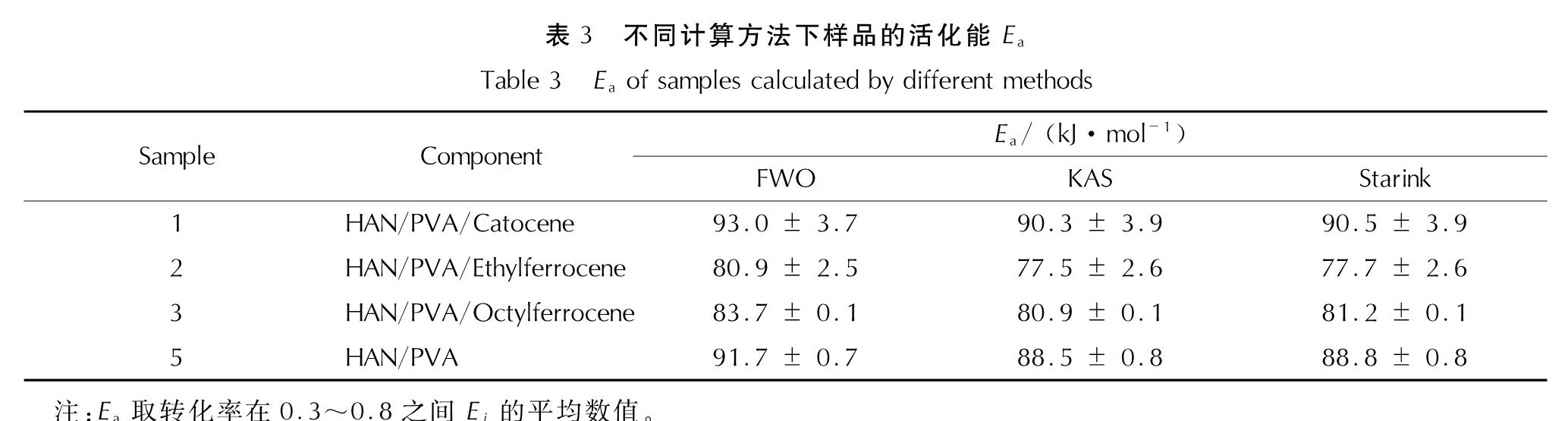
Fig.5 Ei at various values of αi calculated by Starink method由图5可知,4种配方样品的活化能在其热分解过程中基本保持不变,因此样品主反应区的活化能Ea即为各个转化率下活化能的平均值,3种方法的结果见表3。
表3 不同计算方法下样品的活化能Ea
Table 3 Ea of samples calculated by different methods对比表3中数据可知,3种方法计算得到的活化能值相差不大,由于Starink法相对于其他2种方法的准确性较高[22],本研究将Starink法求得的样品活化能作为真值,则Flynn-Wall-Ozawa法和Kissinger-Akahira-Sunose法的相对误差均小于5%。
HAN/PVA样品的指前因子A可由Kissinger方程计算[22]:
In(β/(T2p))=In((AR)/E)-E/(RTp)(5)
式中:Tp为峰值温度,K; f(α)为反应机理函数。
作ln(β/Tp2)-1/Tp关系图,截距即为A。将不同升温速率下样品的Tpi特征值代入公式即求得样品1~样品3和样品5的A分别为4.49×108(±1.72×107)、2.77×105(±9.44×103)、1.53×106(±2.27×103)和1.06×106(±1.00×104)s-1。
由表3可知,卡托辛的加入使得HAN/PVA热分解反应活化能升高,但是图4的TGA-DSC曲线表明其对HAN/PVA的热分解具有明显的催化效果。究其原因,由Arrhenius公式可知:
k=Aexp(-Ea/RT)(6)
式中:k为反应速率常数,s-1; T为热力学温度,K。
样品1~样品3和样品5的反应速率常数k分别为1.34×10-2、2.93×10-4、2.47×10-4和2.11×10-4 s-1。
从式(6)可以看出,k受反应动力学参量Ea和A的影响,虽然HAN/PVA/卡托辛样品的Ea和A均较大,但是其A与HAN/PVA相比提高了2个数量级,k也高出约2个数量级,即A对反应速率的影响远超过Ea的影响,因此卡托辛的加入使HAN/PVA的反应速率常数大幅增加、热分解速率加快,催化效果显著。
3 结 论
(1)HAN/PVA在10K/min条件下热分解的主反应区为457.0~485.2K,失重约73.7%,放热量约为1312.1J/g,主要产物有CO、CO2、N2、NO、NO2、N2O和H2O。
(2)4种二茂铁类衍生物均使得HAN/PVA的热分解温度降低,催化效果明显,催化作用从强到弱依次为叔丁基二茂铁、辛基二茂铁、卡托辛及乙基二茂铁,分别使HAN/PVA的热分解温度降低了约42.9、27.5、17.5和12.3K。
(3)对HAN/PVA/卡托辛、HAN/PVA/乙基二茂铁、HAN/PVA/辛基二茂铁和HAN/PVA样品,3种无模型法计算的活化能近似,相对误差小于5%,热分解反应Ea分别为90.5、77.7、81.2和88.8kJ/mol,A分别为4.49×108、2.77×105、1.53×106和1.06×106 s-1。HAN/PVA/卡托辛样品的热分解反应速率受A的影响远大于Ea,相应的反应速率常数大幅提高,故催化作用显著。
- [1] 汝承博, 许建兵, 代骥, 等. 基于MEMS的固体化学微推进器阵列技术综述[J]. 爆破器材, 2016, 45(6): 1-10.
- [2]林来兴. 现代小卫星的微推进系统[J]. 航天器工程, 2010, 19(6): 13-20.
- [3]SAWKA W N, MCPHERSON M. Electrical solid propellants: a safe, micro to macro propulsion technology[C]∥49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference. San Jose:[s.n.], 2013: 4168.
- [4]曲艳斌, 肖忠良. 硝酸羟胺(HAN)水凝胶性能研究[J]. 含能材料, 2004, 12(3): 168-170.
- [5]何利明, 萧忠良, 刘幼平, 等. 硝酸羟胺水凝胶的贮存性能[J]. 含能材料, 2011, 19(2): 226-228.
- [6]陈军. 火箭发动机燃烧基础[M]. 北京: 北京航空航天大学出版社, 2015: 204-205.
- [7]RAFEEV V A, RUBTSOV Y I.Kinetics and mechanism of thermal decomposition of hydroxylammonium nitrate[J]. Russian Chemical Bulletin, 1993, 42(11): 1811-1815.
- [8]VANDIJK C A, PRIEST R G. Thermal decomposition of hydroxylammonium nitrate at Kilobar pressures[J]. Combustion and Flame, 1984, 57(1): 15-24.
- [9]CRONIN J T, BRILL T B. Thermal decomposition of energetic materials. 8. Evidence of an oscillating process during the high-rate thermolysis of hydroxylammonium nitrate, and comments on the interionic interactions[J]. The Journal of Physical Chemistry, 1986, 90(1): 178-181.
- [10]CRONIN J T, BRILL T B. Thermal decomposition of energetic materials 29 - the fast thermal decomposition characteristics of a multicomponent material[J]. Combustion and Flame, 1988, 74(1): 81-89.
- [11]POPA A F, COURTHEOUX L, GAUTRON E, et al. Aerogel and xerogel catalysts based on θ‐alumina doped with silicon for high temperature reactions[J]. European Journal of Inorganic Chemistry, 2005(3): 543-554.
- [12]KAPPENSTEIN C, BATONNEAU Y, PERIANU E A, et al. Non toxic ionic liquids as hydrazine substitutes. Comparison of physico-chemical properties and evaluation of ADN and HAN[C]∥ Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion. Sardinia: ESA Special Publication, 2004: 557.
- [13]COURTHEOUX L, ROSSIGNOL S,KAPPENSTEIN C, et al. Improvement of catalysts for the decomposition of HAN-based monopropellant-comparison between aerogels and xerogels[C]∥39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit. Huntsville:[s.n.],2003: 4645.
- [14]COURTHEOUX L, POPA F, GAUTRON E, et al. Platinum supported on doped alumina catalysts for propulsion applications. Xerogels versus aerogels[J]. Journal of Non-Crystalline Solids, 2004, 350: 113-119.
- [15]重庆大学. 一种含铝富燃料电点火固体推进剂及其制备方法: CN, CN201610917376.8[P]. 2017-03-15.
- [16] 陈玲红, 陈祥, 吴建, 等. 基于热重-红外-质谱联用技术定量分析燃煤气体产物[J]. 浙江大学学报(工学版), 2016, 50(5): 961-969.
- [17]肖和淼, 杨荣杰, 曾纪朝, 等. 红外光谱研究HNIW热分解残余物的热分解[J]. 火炸药学报, 2012, 35(3): 27-32.
- [18]李彦荣, 赵孝彬, 王宁. 二茂铁衍生物在固体推进剂燃烧过程中的催化机理研究进展[C]∥中国化学会第五届全国化学推进剂学术会议论文集. 大连: 中国化学会, 2011: 301-305.
- [19]FLYNN J H, WALL L A. General treatment of the thermogravimetry of polymers[J]. J Res Nat Bur Stand, 1966, 70(6): 487-523.
- [20]AKAHIRA T, SUNOSE T. Method of determining activation deterioration constant of electrical insulating materials[J]. Res Rep Chiba Inst Technol(Sci Technol), 1971, 16(1971): 22-31.
- [21]STARINK M J. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods[J]. Thermochimica Acta, 2003, 404(12): 163-176.
- [22]胡荣祖, 高胜利, 赵凤起, 等. 热分析动力学(第二版)[M]. 北京: 科学出版社, 2008: 126-127.